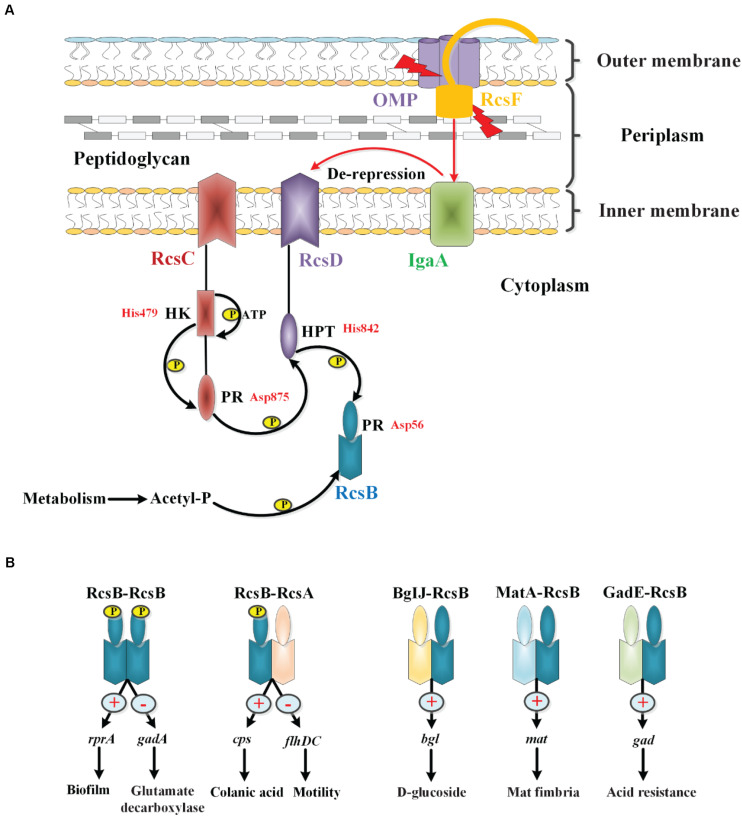
FIGURE 2.
The phosphorylation and regulation of the Rcs system in E. coli. (A) The core components of the Rcs phosphorelay and the transfer of phosphate. RcsF, the OM lipoprotein that senses signals from the OM and periplasm, is seated in an OMP within the OM and is shown interacting with the periplasmic domain of IgaA. IgaA, a five-pass IM protein, is a negative regulator of the phosphorelay. Current model suggests that upon stress signaling, RcsF increases or changes contacts with IgaA, leading to de-repression of the phosphorelay. Then the RcsC autophosphorylates at the HK domain in an ATP-dependent manner. The phosphoryl group is then transferred to the PR domain of RcsC, to the HPT domain of RcsD, and finally to RcsB in a successive manner. In the absence of stress, acetyl phosphate may act as a phosphoryl group donor to maintain a low level of RcsB-P. (B) Regulation of RcsB homodimers and heterodimers. RcsB can form an RcsB-RcsB homodimer or an RcsB-RcsA heterodimer in an RcsB phosphorylation-dependent manner, or form BglJ-RcsB, MatA-RcsB, and GadE-RcsB heterodimers in an RcsB phosphorylation-independent manner, which then interact with a conserved motif in target genes to modulate their transcription, thereby regulating the physiological activities of bacteria.