Figure 1: Flow chart showing study selection as per the MOOSE guidelines.
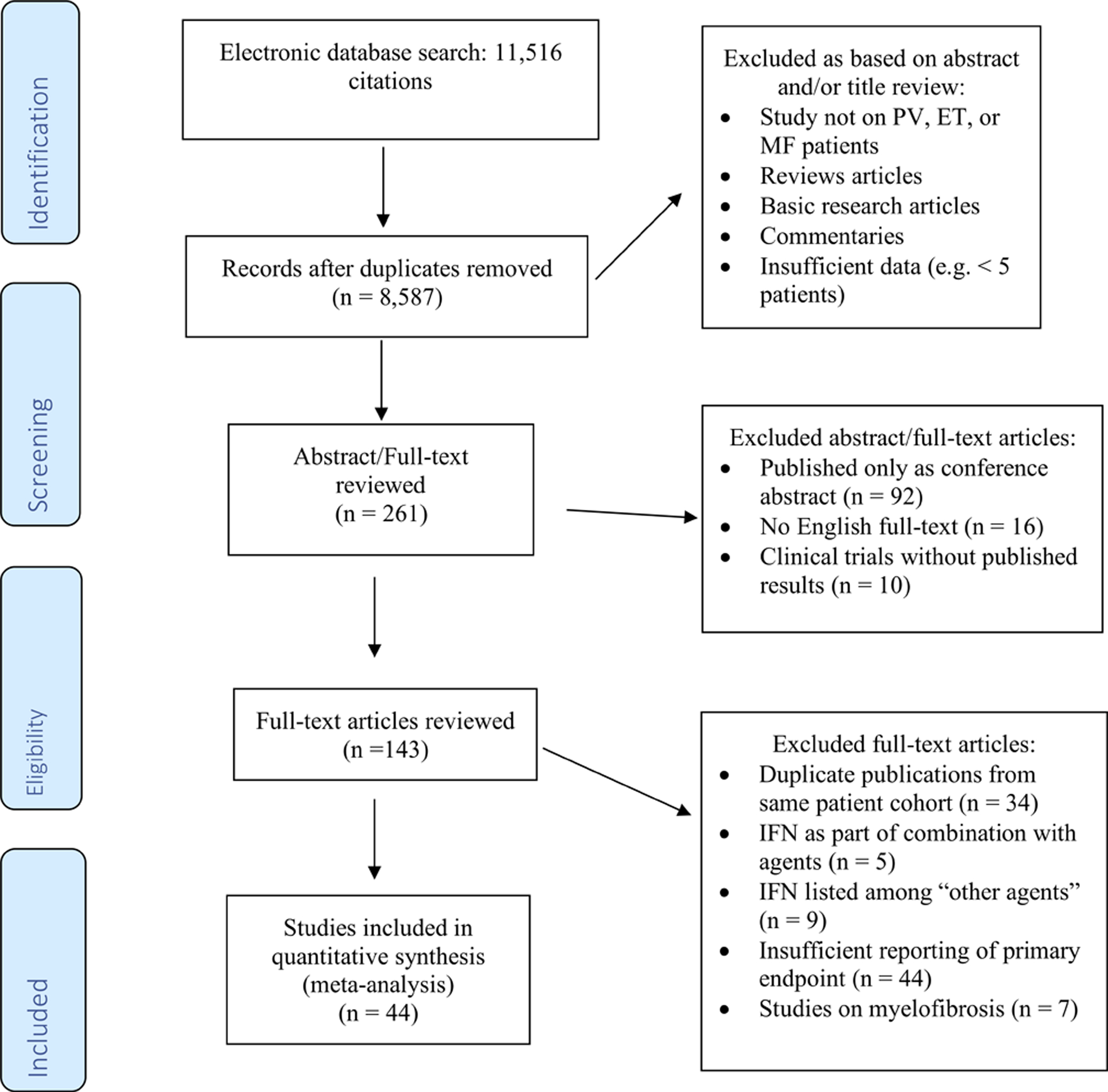
Figure 1 illustrates the search strategy and stepwise process of study selection used in this meta-analysis. MEDLINE and EMBASE via Ovid, the COCHRANE registry of clinical trials (CENTRAL), Scopus and the Web of Science electronic databases were searched without language restriction from inception through March 21st, 2019, using the following combination of free-text terms linked by Boolean operators: [“polycythemia” OR “polycythemia vera” OR “essential thrombocytosis” OR “essential thrombocythemia” OR “myelofibrosis” OR “myeloproliferative neoplasm” OR “MPN”] AND [“interferon” OR “IFN” OR “pegylated interferon” OR “peginterferon” or “alpha2b interferon” OR “alpha2 interferon” OR “alpha interferon”]. After removal of duplicates, two authors (JPB and MS) independently screened the titles and abstracts of all retrieved studies for eligibility. Studies were excluded if they were (I) review articles, commentaries or basic research articles, (II) reporting results from diseases other than ET, PV, or MF (e.g. chronic myeloid leukemia), or (III) case series with less than 5 patients. Subsequently, full texts of the potentially eligible studies were reviewed for eligibility. We excluded studies that 1) lacked information on the primary outcome of overall response rates, 2) IFN listed only among “other therapies” without separate reporting of outcome data, 3) studies published only in abstract form, 4) duplicate publications from the same patient cohort, 5) IFN given as part of combination therapy, 6) clinical trials without published results, and 7) without an available English full text. Studies on myelofibrosis have been reported separately and were excluded from this meta-analysis.
