Table 1.
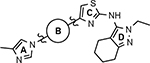
B-ring analogs of GSM 4.
 | ||||
|---|---|---|---|---|
| Cmpd | B-ring | Aβ42 IC50a | clogPb | Kin. Aq. Sol.c |
| 4 | 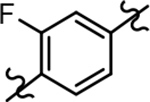 |
163 ± 10 | 3.88 | ntd |
| 22a | 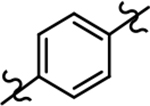 |
660 ± 131 | 3.74 | ntd |
| 22b | 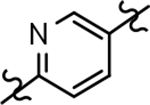 |
>1000 | 3.13 | ntd |
| 22c | 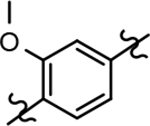 |
171 ± 67 | 3.49 | 4.6 |
| 22d |  |
60 ± 15 | 3.28 | 4.5* |
| 22e | 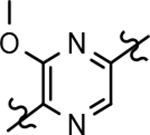 |
89 ± 38 | 2.66 | <1.6 |
| 23 | 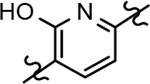 |
468 ± 165 | 3.25 | ntd |
IC50 represents the concentration in nM of compound required for reducing Aβ42 levels by 50%. The IC50 values are the mean ± standard deviation of at least 2 determinations.
Calculated partition coefficient of the simulated ratio of the compound’s concentration in octanol to the compound’s concentration in water using ChemAxon fragment based approach.
Kinetic aqueous solubility measured at pH 7.4 by UV/Vis absorbance in PBS buffer (μM).
nt = not tested - compound did not meet minimum activity threshold.
