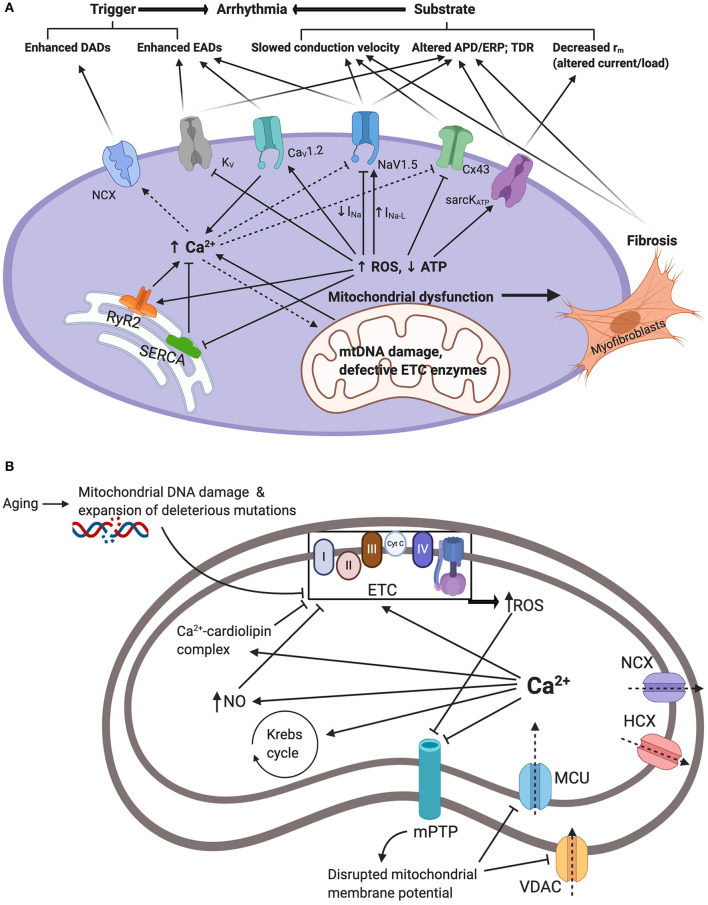
Figure 1.
(A) Summarizes the interacting mechanisms by which mitochondrial dysfunction promotes arrhythmogenesis. Mitochondrial dysfunction driven by mitochondrial DNA damage and defective electron transport chain (ETC) enzymes results in reduced ATP and increased reactive oxygen species (ROS) production. In turn, this modifies function and/or expression of sarcolemmal ATP-sensitive K+ channels (sarcKATP), gap junction proteins (Cx43), cardiac sodium channels (NaV1.5), cardiac L-type voltage gated Ca2+ channels (CaV1.2), and cardiac voltage gated potassium channels (KV). Moreover, ROS modify endoplasmic reticulum Ca2+ homeostasis proteins ryanodine receptor (RyR) and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) resulting in elevated cellular Ca2+ levels which drives the depolarizing Na+-Ca2+ exchanger (NCX). Together, these changes increase arrhythmic triggers and substrates. APD/ERP: action potential duration/effective refractory period ratios; DADs: delayed after-depolarizations; EADs: early after-depolarizations; TDR: transmural dispersion of repolarization. (B) Summarizes mitochondrial Ca2+ handling and its relation to mitochondrial dysfunction and elevated cytosolic Ca2+. Ca2+ enters the mitochondria via voltage dependent anion channels (VDAC) and mitochondrial calcium uniporter (MCU). This is driven by the negative mitochondrial membrane potential (~−180mV). Increased cytosolic Ca2+ through mechanisms demonstrated in (A) will result in increased uptake and concentration of mitochondrial Ca2+. Mitochondrial Ca2+ overload contributes to mitochondrial dysfunction. Firstly, Ca2+ stimulation of the Krebs cycle and the oxidative phosphorylation electron transport chain increasing electron leakage and ROS by-products. Secondly, Ca2+-cardiolipin complexation disrupting mitochondrial lipid and protein arrangement causing proteins such as cytochrome c dislocation and inhibition of the electron transport chain and ROS production. Thirdly, through activation of nitric oxide synthase generating NO radicals which inhibit components of the ETC and promote ROS production. Increased ROS and cytosolic Ca2+ also inhibit mitochondrial permeability transition pores (mPTP) impairing Ca2+ uptake and contributing to increased cytosolic Ca2+. Ca2+ is also extruded through the hydrogen-calcium exchanger (HCX) and the mitochondrial sodium-calcium exchanger (NCX).