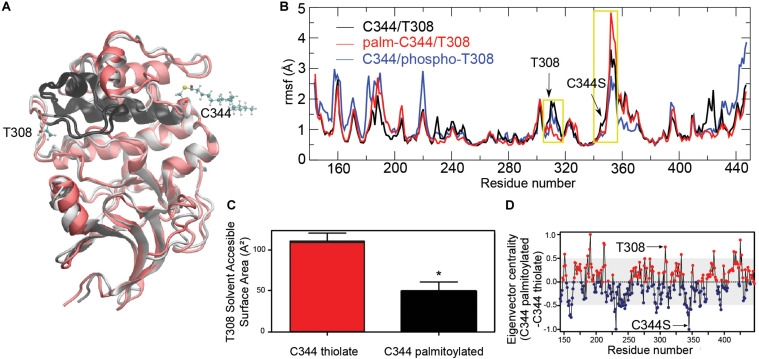
FIGURE 2.
Effect of S-palmitoylation on Akt 3D structure. (A) Alignment of unmodified (gray) and S-palmitoylated (red) representative structures of apo-Akt1 model. C344, T308 and palmitoyl group are depicted in balls and cylinders for the S-palmitoylated protein. Protein backbone connecting T308 and C344 is highlighted in black. (B) Root mean square fluctuation (rmsf, Å) per residue for unmodified Akt1 (black), palmitoylated-C344 Akt1 (red) or phospho-T308 Akt1 (blue). Yellow boxes highlight T308 and C344 areas. (C) Comparison of solvent accessible surface area (SASA, Å2) of T308 obtained from unmodified (black) and S-palmitoylated (red) MD simulations. *p < 0.01 compared to C344 thiolate (Student’s t-test). (D) Eigenvector centrality measurement of mutual information for characterization of T308-C344 structural connection.