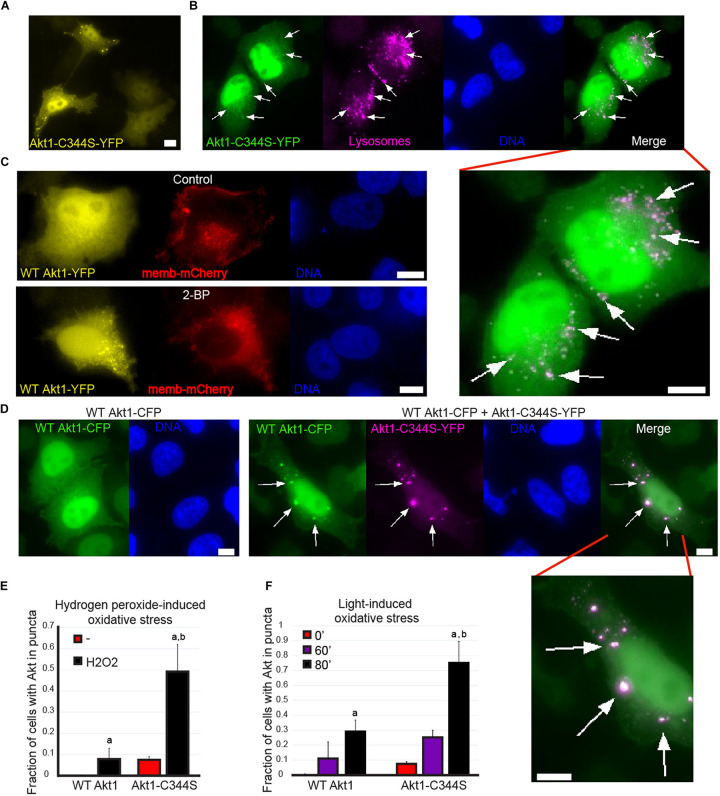
FIGURE 4.
A link between Akt S-palmitoylation, lysosome recruitment and the response to oxidative stress. (A) An example of untreated cells displaying Akt1-C344S-YFP in cytoplasmic puncta; scale bar, 5 μm. (B) HeLa Kyoto cells were transfected with a plasmid coding for Akt1-C344S-YFP (green) and live stained with Lysotracker Red to observe lysosomes (magenta). DAPI was used to reveal cell nuclei (blue); scale bar, 5 μm. The Pearson’s correlation coefficient was calculated in different ROI (marked with arrows) obtaining an average of 0.71 +/– 0.12 between Akt1-C344S-YFP and Lysotracker Red (as a control, a correlation coefficient of 0.17 +/– 0.28 was obtained when comparing Akt1-C344S-YFP and DNA). (C) HeLa Kyoto cells were co-transfected with plasmids coding for a PM fluorescent marker (pCS-memb-mCherry, red) and WT Akt1-YFP (yellow). 48 h later, cells were treated with 100 μM of the palmitoylation inhibitor 2-BP or DMSO (vehicle) for 1 h and fixed. A significant increase in the proportion of cells displaying WT Ak1-YFP in puncta (one at least) was found when we treated them with 2-BP (from 1% in control to 30% in cells treated with 2-BP, p < 0.05 according to Student’s t-test). PM localization of pCS-memb-mCherry depends on the palmitoylable N-terminal sequence of Lyn kinase and therefore it was used as a positive control of the effect of 2-BP. DAPI was used to reveal cell nuclei (blue); scale bar, 5 μm. (D) HeLa Kyoto cells were transfected with a plasmid coding for Akt1-C344S-YFP (magenta) and/or a plasmid coding for WT Akt1-CFP (green). After 48 h, cells were fixed. Left: normal WT Akt1-CFP localization. Right: Localization of WT Akt1-CFP in cells with Akt1-C344S-YFP puncta. DAPI was used to reveal cell nuclei (blue); scale bar, 5 μm. (E) HeLa Kyoto cells were transfected with a plasmid coding for either WT or Akt1-C344S-YFP. Forty-eight hours later, cells were incubated for 3 h in the presence or absence of 10 mM hydrogen peroxide, fixed, and cells were scored as having or not YFP signal in puncta. Data in the plots corresponds to the fraction of cells containing YFP in puncta (mean +/- SEM). Letters indicate significant differences (ANOVA). a. p < 0.05 compared to untreated cells expressing the same version of Akt. b. p < 0.05 compared to WT Akt1-YFP cells under the same treatment. (F) HeLa Kyoto cells were transfected with a plasmid coding for either WT or Akt1-C344S-YFP. Live cells were imaged every 20 min to deliberately induce photo-damage. At every time point, we performed a Z Series with 3 z steps of 1 μm for 3 different wavelengths (RFP, exposure: 0.2 s; YFP, exposure: 0.5 s; CFP, exposure: 0.1 s) and cells were scored as having or not YFP signal in puncta. Data in the plots corresponds to the fraction of cells containing YFP in puncta (mean +/- SEM). Letters indicate significant differences (Student’s t-test): a. p < 0.05 compared to the same cells at t = 0’. b. p < 0.05 compared to WT Akt1-YFP cells at t = 80’.