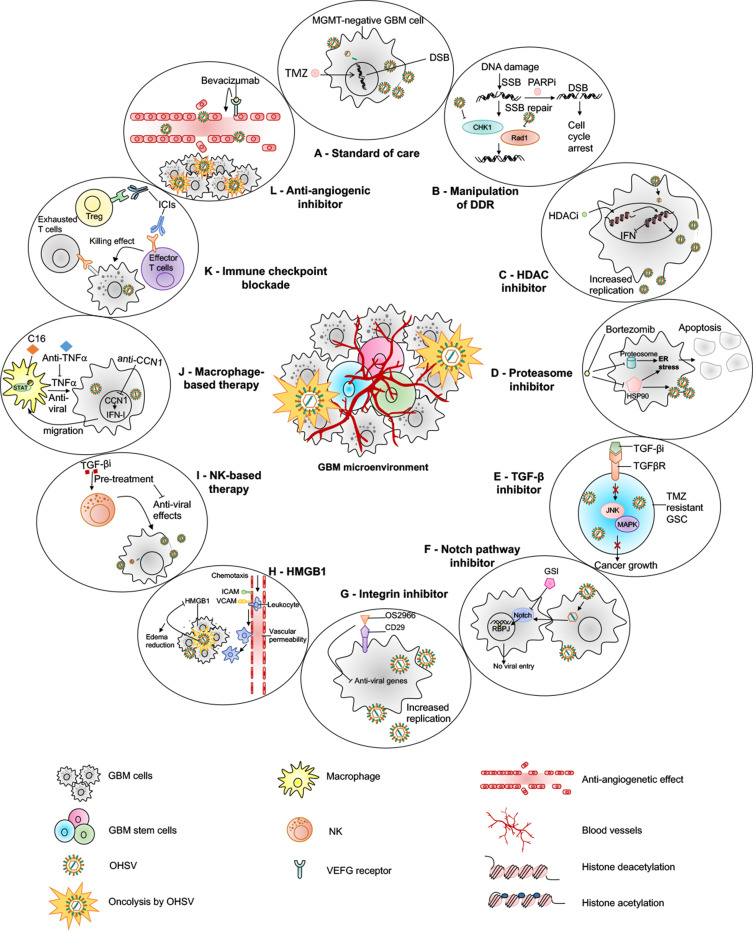
Figure 3.
oHSV-based combination therapies for GBM. This figure represents strategies to combine oHSV with different anti-cancer therapies for GBM treatment. (A) oHSV can be synergistically combined with TMZ, a DNA-alkylating agent and an immunomodulator, to induce DNA damage response in MGMT-negative GBM cells. (B) oHSV replication promotes degradation of Rad51 and Chk1 whose functions are important for SSB repair mechanism. Inhibition of PARP in DNA-damaged cells facilitates the conversion of SSB to DSB. The combination of oHSV and PAPRi synergistically induces cell cycle arrest in GBM. (C) HDAC is an enzyme that controls cancer cell survival/progression and upregulates IFN genes. Treatment with HDAC inhibitors prior to oHSV infection inhibits induction of anti-viral IFN genes, resulting in increased transcription of viral genes and improved virus replication. (D) Bortezomib is a proteasome inhibitor, which induces unfolded protein response (UPR), characterized by induction of heat-shock proteins (HSP) 40, 70 and 90, and ER stress in cancercells. Bortezomib-induced ER stress and UPR significantly enhance oHSV replication and synergistic killing of GBM cells. (E) TGF-β plays a critical role in GBM pathogenesis and in maintaining the stemness of GSCs. In TMZ-resistant GSC model, the combination of oHSV and TGF-βi synergistically kills TMZ-resistant recurrent GSCs, increases oHSV replication and induces JNK-MAPK signaling blockade, and eventually inhibits tumor progression. (F) oHSV infection of tumor cells leads to activation of notch signaling in adjacent non-infected tumor cells. Notch signaling pathway plays a critical role in cell-cell interaction and viral spread. Inhibition of notch signaling pathway such as GSI results in increased killing of GBM cells after oHSV therapy. (G) ITGB1, also referred as CD29, plays a critical role in tumor cell proliferation and progression. OS2966 (a humanized CD29 blocking antibody) blocks CD29, reduces the expression of anti-viral genes (IFNα, IFNβ, Stat1, OAS1, OAS2, IRF3, IRF9, and PKR), suppresses oHSV-induced macrophage activation, resulting in enhanced oHSV replication and oncolysis. (H) GBM cells that are infected by oHSV upregulate HMGB1. HMGB1 causes upregulation of ICAM and VCAM, increases vascular permeability and PMBC infiltration to the tumor, leading to edema that might cause CNS injuries. Thus, combination of anti-HMGB1 and oHSV increases survival by reducing brain injuries. (I) Recruitment of NKs after oHSV administration can limit oHSV replication and oHSV-mediated anti-tumor efficacy. Transient inhibition of anti-viral effects of NKs by TGF-β inhibitors enhances viral replication and viral yield. (J) Similarly, transient blockade of TNFα, produced from anti-viral macrophages, by TNFα blocking antibodies or inhibition of STAT1/3 phosphorylation by C16 enhances oHSV replication. In addition, virus-infected cells upregulate CCN1, which in turn activates an intracellular type I IFN response and increases infiltration of macrophages to the site of infection. Treatment with anti-CCN1 reduces virus clearance by macrophages, resulting in better anti-tumor efficacy. (K) Administration of immune checkpoint blockade such as anti-PD-1, anti-PD-L1, anti-CTLA4 prevents T cell exhaustion and enhances oHSV-mediated-anti-tumor immunity. (L) Bevacizumab binds to VEGF, reduces tumor vascularization, decreases vascular permeability, and inhibits tumor growth; however, it also induces tumor cell invasion. An oHSV expressing anti-angiogenic vasculostatin that contains an integrin-antagonizing RGD (Arg-Gly-Asp) motif significantly inhibits glioma cell migration/invasion following bevacizumab treatment, leading to a significant extension of survival compared to bevacizumab monotherapy.
Abbreviations: TMZ, temozolomide; MGMT, O-6-methylguanine-DNA methyltransferase; DSB, double-strand break; SSB, single-strand break; PARP, poly(ADP-ribose) polymerase; DDR, DNA damage response; HDAC, histone deacetylase; IFN, interferon; UPR, unfolded protein response; ER, endoplasmic reticulum; TNF-α, tumor necrosis factor-alpha; TGF-β, transforming growth factor-beta; GSC, glioma stem cells; RBPJ, recombination signal binding protein for immunoglobulin kappa J region; JNK, c-Jun NH2-terminal kinases; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; MAPK, mitogen-activated protein kinase; GSI, gamma-secretase inhibitor; ITGB1, integrin β1; OAS1, 2ʹ-5ʹ-Oligoadenylate Synthetase; IRF, interferon regulatory factor; PKR, protein kinase R; HMGB1, high mobility group box 1; CNS, central nervous system; STAT, signal transducer and activator of transcription; CCN1, cellular communication network factor 1; PD-1, programmed death-1; PD-L1, programmed death ligand-1; CTLA4, cytotoxic T-lymphocyte-associated protein 4.