Abstract
We examined the effect of the Qi-invigorating Traditional Chinese Medicines (TCM) herb Panax ginseng (P.G.) on mitochondrial functions and cellular antioxidant capacity in different organs of mice. We found that the P.G. extracts had a significant effect on tissues of mice, with the generation of total adenylate pool (TAP) enhanced in all visceral tissues, but not for the brain. The mitochondrial membrane potential (MMP) and antioxidant capacity reflected by superoxide dismutase (SOD) and glutathione (GSH) increased only for the meridian tissues that P.G. belongs to including Heart, Spleen and Lung. Reactive oxygen species (ROS), as a combined result of the increased energy metabolism and antioxidant capacity, varied in different organs. We concluded that: 1) the Qi-invigorating TCM herb P.G. had a significant effect on mice by enhancing TAP production in all of the visceral tissues examined, except for the brain; 2) for the meridional tissues of P.G. (Heart, Spleen and Lung), the P.G. extracts not only promoted the TAP production, but also boosted the antioxidant capacity demonstrated by the simultaneous increase in TAP, and SOD and GSH.
Keywords: Qi, mitochondria, adenosine triphosphate, antioxidant capacity, meridian tropism
Introduction
Qi is the most important concept in TCM theory, which stimulates the flow of blood throughout the body, and promotes the absorption and utility of the nutrients of food. Mitochondria are considered to be the engine of life with the main function of providing ATP for cellular activities through oxidative phosphorylation.1 Qi in the TCM system and mitochondria in modern medicine system play similar roles in providing power for cells and promoting the transformation of material and energy,2 and therefore, a connection between the mitochondria and Qi has been suggested.3–5
Studies have shown that Qi-invigorating herbs including Panax ginseng can affect mitochondria energy production6 and antioxidant capacity.7 The active ingredients such as polysaccharide and ginsenoside in Panax ginseng extracts played an important role in alleviating cellular oxidative stress through either the reduction in ROS levels,8 or protection from lipid peroxides and increase in SOD levels.9,10
The theory of “meridian tropism” refers to the selective action of a drug on a certain part of the body, that is, a drug has a special affinity to certain internal organs, on which it has a major or special therapeutic effect. Panax ginseng whose characteristics are sweet, slightly bitter, and lukewarm, belongs to the Lung, Spleen and Heart meridians. Panax ginseng has the functions of tonifying vitality, invigorating the Spleen and Lung, calming the mind, and promoting intelligence.11 Previous studies found that ethanolic extract of Panax ginseng significantly enhanced the growth of primary spleen cells in the spleen, and boosted ATP production of Caco-2 colonic epithelial cells in vitro which is consistent with the TCM meridian theory that the Spleen meridian includes the function of both the spleen and the intestinal system.12
The hypothesis of this study is that Qi-invigorating herbs 1) boost ATP production in mitochondria and 2) increase the antioxidant capacity simultaneously for specific organs of animals based on the meridians it belongs to. To examine this hypothesis, we conducted animal experiments to study the effect of Panax ginseng extracts on various tissues including heart, hiver, spleen, sung, kidney, brain and skeletal muscle tissues, and compared the energy metabolism and antioxidant changes of mitochondria in lung, spleen and heart tissues with those in other tissues. Our goal was to further explore the mechanism of Panax ginseng’s role in the supplementation of Qi in the context of the meridian tropism theory and to explain the biochemical nature of “Qi” in TCM.
Methods
Herbal Preparation
The herb materials Panax ginseng was purchased at a Chinese medicine store (Tongrentang chain-store), and the herbal extract was prepared as follows: Panax ginseng (100 g) was processed by cutting into small pieces first, followed by extracting under reflux in 500 mL 95% ethanol at 65 °C for 2 h, and this procedure was conducted twice. The solvent in the combined extract was evaporated under reduced pressure, generating an ethanoic extract of Panax ginseng at the yield of 5% (w/w).
Animal Care and Treatment
Mice used in the experiment were purchased from Beijing Vital River Laboratory Animal Technology Co. Adult female C57BL/6J mice (8 months; 35 ± 2 g; Specific Pathogen Free) were fed ad libitum with food and water, and maintained at the condition of a 22 °C with a 12 h dark/light cycle in the Animal Care Facilities. Animals were divided into 2 groups, with 3 in each. For the animals in the treatment group, they were intragastrically administered with Panax ginseng extracts which was dissolved in distilled water (0.05 g/mL) at a daily dosage of 0.20 mL/10 g for 7 days. Animals in the control group received distilled water only. At 24 h after the last dosing, the mice were anaesthetized with isoflurane gas. The organs including the heart, liver, spleen, lung, kidney, brain and skeletal muscle (hind limb) tissues were excised and were stored in liquid nitrogen.
Preparation of Tissue Homogenates
Minced different tissue samples were homogenized in ice-cold PBS (1/10, g/mL) with a Teflon-glass homogenizer. After that, tissue extract went through centrifugation (3,000 g at 4 °C) for 5 min to remove cell debris, and the tissue homogenates obtained were measured for ATP/ADP/AMP, SOD and GSH levels.
Procedure for Mitochondrial Fractioning
Tissue homogenates (200 μL) were centrifuged at 1,500 g at 4 °C for 20 min to obtain mitochondrial pellets, and then the supernatant was centrifuged at 17,000 g at 4 °C for 20 min to sediment the mitochondria. After that, 400 μL buffer (consisting of 210 mM Mannitol, 70 mM sucrose, 10 mM MgCl2, 5 mM K2HPO4, 1 mM EGTA, 10 mM Tris-base, PH 7.4) was added to the mitochondrial pellets to reconstitute the mitochondrial fractions, which were subjected to measurement of ROS and MMP levels.
Biochemical Analysis
Measurement of ATP, ADP, and AMP levels
A new HPLC analysis was employed to study ATP, ADP, and AMP in tissue homogenates.13 An aliquot of 1 mL tissue homogenates were sampled to collect cells by centrifugation at 10,000 g for 5 min, followed by adding 100 µL PBS and 40 µL deionized water. After that, 360 µL perchloric acid (6%) was added to remove the protein by keeping samples on ice for an additional 10 min. The cell extract was centrifuged at 10,000 g at 4 °C for 5 min, with 300 µL supernatant neutralized with 40 µL of 2 M K2CO3, and filtered through a 0.45 µm filter. A 10 µL of neutralized cell extract was used for the measurement of ATP, ADP, and AMP using a HPLC with mobile phase (0.1 M KH2PO4 buffer at pH 6.25) and 5% methanol (v/v) set at a rate of 0.6 mL/min and a detection wavelength of 254 nm. The quantitation of the 3 components was done by computing peak areas and injecting standard solutions of nucleotides with known concentrations. Total adenylate pool (TAP) was the sum of all 3 components ([TAP]=[ATP]+[ADP]+[AMP]). Finally, TAP levels in cells were normalized to the total cell protein.
Measurement of ROS levels
ROS levels in tissue homogenates were determined as described by Leung et al.14 In brief, 50 µL aliquots of the mitochondrial fraction and 60 µL DCFH-DA solution (2 μM) were added into wells of a micro-titer plate to allow the mixture to incubate at 37 °C for 10 min under dark conditions, followed by adding 50 µL of incubation buffer (0.1 mM EGTA, 5 mM KH2PO4, 3 mM MgCl2, 145 mM KCl, 30 mM Hepes, pH 7.4) and 50 µL of substrate solution (20 mM pyruvate and 10 mM malate). The reaction mixture was subject to the measurement of fluorescence intensity (excitation of 485 nm, emission of 535 nm) every 5 min for 60 min. Mitochondrial ROS levels were obtained from the fluorescence intensity of the sample after subtracting the value of a blank sample containing incubation buffer, substrate solution, and DCFH-DA.
Mitochondrial membrane potential measurement
Mitochondrial membrane potential (MMP) was quantified in accordance with the protocol of the Mitochondrial Membrane Potential Assay Kit (Product No.: C2006; Beyotime, China). To study the MMP levels in tissue homogenates, 50 µL aliquots of the mitochondrial fractions were incubated with a JC-1 probe at 37 °C for 20 min in the dark. After the incubation, cells were washed with ice-cold JC-1 buffer solution twice. The JC-1 probe entering the mitochondrial matrix will aggregate and emit at 590 nm (red) upon excitation at 525 nm, whereas the monomeric JC-1 remaining in the cytoplasm emits at 530 nm (green) upon excitation at 490 nm. The fluorescence intensity was recorded using a microplate reader (Tecan, Switzerland), with the value of MMP calculated by the ratio of red to green.
Measurement of antioxidant levels
Antioxidant levels in the different tissue homogenates were evaluated by the enzyme activities of SOD and GSH using an SOD Assay Kit with WST-8 (Product No.: S0101; Beyotime, China) and a total Glutathione Assay Kit (Product No.: S0052; Beyotime, China) following the manufacture’s protocols. The absorbance of SOD and GSH was assessed at 450 nm and 412 nm respectively using a microplate reader and the SOD and GSH levels in cell homogenates were normalized to total cell protein.
Statistical Analysis
Values of the different measurement were normalized to a respective mean control value from control samples and expressed as percent control. All data is expressed as mean ± standard deviation (SD). They were analyzed by analysis of variance (ANOVA) and Least Significant Difference (LSD) using GraphPad InStat software, where P < 0.05 was considered statistically significant.
Results
Feeding Panax ginseng extracts to mice significantly increased the TAP content in most organs including heart, liver, spleen, lung, kidney and skeletal muscle, except for the brain as shown in Figure 1A. ROS level varied in the organs examined, with a significant reduction in heart, lung, kidney and brain, an increase in the spleen tissues, and unchanged for the liver and skeletal muscle tissues (Figure 1B). While MMP significantly increased in the meridian organs of Panax ginseng including heart, spleen, and lung of mice, and maintained unchanged in the other in other organs, i.e. liver, kidney, brain, muscle (Figure 1C).
Figure 1.

Effect of Panax ginseng (P.G.) extract on mitochondrial functions in various tissues of mice. (A) TAP; (B) ROS; (C) MMP. Animals were orally treated with P.G. at the indicated daily doses for 7 days. Data were expressed in percentage with respect to the control. *P < 0.05; **P < 0.01, when compared with the untreated control.
Antioxidant capacity of the animals was evaluated by measuring SOD activity and GSH levels. As shown in Figure 2, both SOD and GSH increased significantly in the meridian organs of Panax ginseng (i.e. heart, lung and spleen). SOD in other tissues showed no significant changes (Figure 2A), whereas GSH decreased in the tissues of kidney and brain (Figure 2B).
Figure 2.
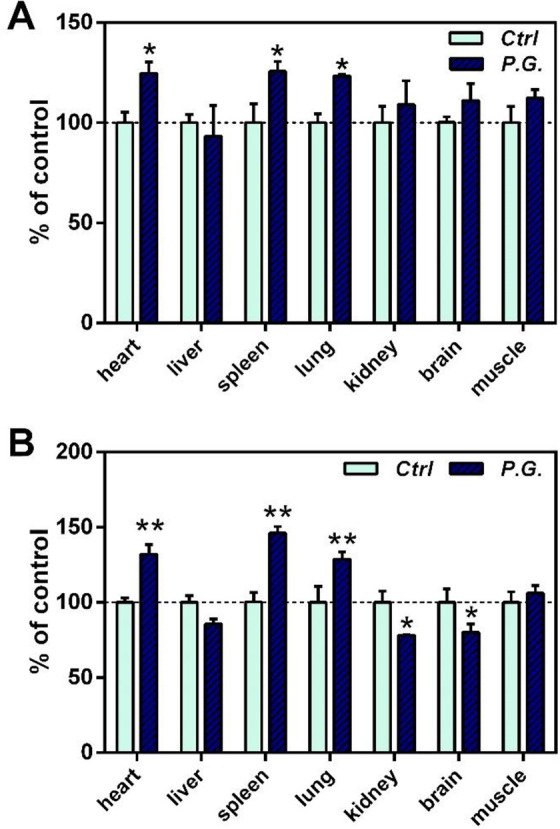
Effect of Panax ginseng (P.G.) extract on antioxidant capacity in various tissues of mice. (A) SOD; (B) GSH. Animals were orally treated with P.G. at the indicated daily doses for 7 days. Data were expressed in percentage with respect to the control. *P < 0.05; **P < 0.01, when compared with the untreated control.
Compared with the control mice, the changes in the weight of the organs after feeding Panax Ginseng had no significant difference (Figure 3).
Figure 3.
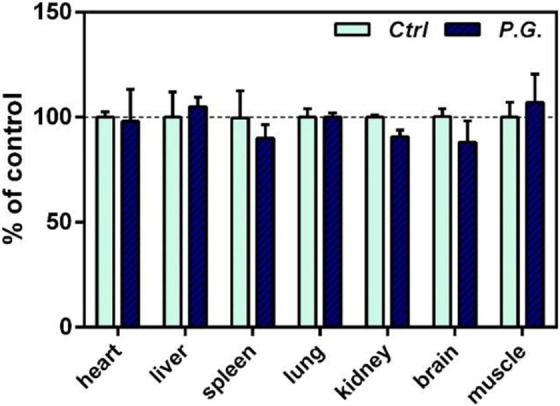
Effect of Panax ginseng (P.G.) extract on organ weight in various tissues of mice. Animals were orally treated with P.G. at the indicated daily doses for 7 days. Data were expressed in percentage with respect to the control. *P < 0.05; **P < 0.01, when compared with the untreated control.
Discussion
In TCM theory, Qi is considered the most basic and important energy that constitutes the human body and supports life activities with numerous critical functions.15 Qi dominates all of life’s activities, regulating yin-yang balance and the 5 elements, nourishing the internal organs, transforming water and grain, and imbibing the essence of blood. Qi keeps moving around the body to promote and regulate the metabolism maintaining overall life processes. A movement imbalance of Qi in TCM causes pathological states of the body.
The main function of mitochondria in eukaryotic cells is to carry out aerobic respiration and energy metabolism, providing ATP for cellular functions. During this process, ROS is produced as a co-product due to electron leakage at various sites on the electron transfer chain (ETC), which can cause serious damage to cells when over produced.16 In addition to performing energy metabolism, mitochondria also regulate cell apoptosis and participate in almost all REDOX reactions in the body, providing defense and regulatory effects.17 Qi and mitochondria are very similar in function. At present, studies have shown that mitochondria are closely related to Qi,3–5 and it has been found that Qi-invigorating herbs can enhance the production of ATP.7
Panax ginseng can preferentially act on the “Heart” “Spleen” and “Lung” based on the “meridian tropism” theory.11 Studies have shown that Panax ginseng can increase the primary spleen cell proliferation and boost the ATP production in the colonic epithelial cells of Caco-2 cultured in vitro,12 which is consistent with the TCM theory that the Spleen meridian includes both the spleen and the intestinal system.11 Other study also demonstrated significant increases in ATP in H9c2 cardiomyocytes in vitro when supplemented with Panax ginseng.6
For this study, we comprehensively evaluated the changes at the mitochondrial level of different tissues as well as the antioxidant capacity of tissues in mice after feeding Panax ginseng ethanoic extract, and compared the changes of these parameters between the meridian organs that Panax ginseng belongs to (Heart, Spleen, Lung) and regular tissues (liver, kidney, brain and skeletal muscle). We found that the Qi-invigorating herb, Panax ginseng increased the energy metabolism as reflected by the increasing TAP for all viscera but not for the brain, which we speculate could be related to the blood-brain barrier. However, accompanying the increasing mitochondrial energy metabolism, the MMP and the antioxidant capacity as demonstrated by SOD and GSH increased only in the meridian organs that Panax ginseng acts on including Heart, Spleen and Lung, but not for the other organs. It is critical to have enhanced antioxidant capacity to suppress the ROS generated along with the increased TAP production, so the improved energy metabolism can be sustained. Based on this finding, we propose that the biochemical basis of meridian tropism for Qi-invigorating herbs is to simultaneously enhance both the mitochondrial energy metabolism and the antioxidant capacity of the organs it belongs to.
As a by-product of ATP, the ROS normally increases with the increasing generation of ATP.18,19 However, the Qi-invigorating herbs tends to increase both the ATP and the antioxidant capacity.20 Consequently, ROS, as the combined effect of these 2 factors, could be either lower or higher than that of the control. In our study, ROS was lower than the control for most of the organs, although, the ROS in the spleen tissue was significantly higher than the control despite the increase in GSH and SOD in this tissue.
In conclusion, our results showed that the Qi-invigorating Panax ginseng extracts can enhance the mitochondrial energy metabolism in general. However, for its meridian organs based on the tropism theory, P.G. enhanced both the energy production and antioxidant capacity simultaneously, which we propose is the biochemical basis of the meridian tropism theory for this Qi-invigorating herb.
Acknowledgements
The authors would like to thank the ENN Group for the funding.
Authors’ Note: Mengmei Li and Yu Chen contributed equally to this work. Research design: Xuemei Bai, Bruce Qing Tang; Methodology: Yu Chen, Mengmei Li, Qian Feng, Jie Teng, Lin Wang; Experiments: Yu Chen, Mengmei Li, Zhongzhen Cai, Qian Feng, Jie Teng, Yuming Chen, Lin Wang, Caixia Li; Data Analysis: Yu Chen; Writing-Original Draft Preparation: Mengmei Li, Yuming Chen; Writing-Review & Editing: Xuemei Bai, Yu Chen. All authors discussed the results. All animal treatments in this research were performed in accordance with international ethical guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental protocols were approved by Committee of Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ENN Research Fund.
ORCID iD: Yuming Chen, PhD  https://orcid.org/0000-0003-0423-2274
https://orcid.org/0000-0003-0423-2274
References
- 1. Yao W, Yang H, Ding G. Mechanisms of Qi-blood circulation and Qi deficiency syndrome in view of blood and interstitial fluid circulation. J Tradit Chin Med. 2013;33(4):538–544. [DOI] [PubMed] [Google Scholar]
- 2. Cornelius C, Perrotta R, Graziano A, Graziano EJ, Graziano V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: mitochondria as a “chi”. Immun Ageing. 2013;10(1):15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace DC. Mitochondria as chi. Genetics. 2008;179(2):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang ML, Zhang LT, Qiu XF, et al. Discussion on the relationship of mitochondrion and qi. Chinese J Basic Med TCM. 2001;7(4):60–61. [Google Scholar]
- 5. Lin F, Guo LL, Wang J. Expounding the Functions of Qi in TCM based on the effect of mitochondria. Chin J Integrated Tradit Western Med. 2014;34(8):903–905. [PubMed] [Google Scholar]
- 6. Wong HS, Cheung WF, Tang WL, Ko KM. “Qi-Invigorating” Chinese tonic herbs (Shens) stimulate mitochondrial ATP generation capacity in H9c2 cardiomyocytes in situ and rat hearts ex vivo. Chin Med. 2012;3(2):101–105. [Google Scholar]
- 7. Ko KM, Leung HY. Enhancement of ATP generation capacity, antioxidant activity and immunomodulatory activities by Chinese Yang and Yin tonifying herbs. Chin Med. 2007;2(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tian YB, Zhao DQ, Yan LX. Protective effect of ginseng polysaccharides on oxidative stress injury in cardiomyocytes by reducing ROS level and apoptosis. J Central China Normal Univ (Nat Sci). 2018;52(2):91–98. [Google Scholar]
- 9. Wang M, Ke Y, Dan Z. Effect of panaxadiol saponins on LPO, SOD in swimming training rats. J Norman Bethune Univ Med Sci. 2001;27(4):358–360. [Google Scholar]
- 10. Kim YH, Park KH, Rhoxy HM. Transcriptional activation of the Cu, Zn-superoxide dismutase gene through the AP2 site by ginsenoside Rb2 extracted from a medicinal plant, Panax ginseng. J Biol Chem. 1996;271(40):24539–24543. [DOI] [PubMed] [Google Scholar]
- 11. Gao XM. Chinese Material Medicinea. China Press of Traditional Chinese Medicine; 2007. [Google Scholar]
- 12. Leong PK, Leung HY, Chan WM, et al. Pharmacological Investigation of “Meridian Tropism” in Three “Shen” Chinese herbs. Chin Med. 2019;10(4):121–135. [Google Scholar]
- 13. Chen YJ, Wu XX, Li TJ. A method for detecting the energy of organism: China, 11422682 .5 [P]. 2017. 2018-07-20. [Google Scholar]
- 14. Leung HY, Chiu PY, Poon MK, Ko KM. A Yang-invigorating Chinese herbal formula enhances mitochondrial functional ability and antioxidant capacity in various tissues of male and female rats. Rejuvenation Res. 2005;8(4):238–247. [DOI] [PubMed] [Google Scholar]
- 15. Yin HH. The Basic Theories of Traditional Chinese Medicine. Shanghai Scientific & Technical Publishers; 1984. [Google Scholar]
- 16. Xu JX. Principle of Health and Longevity in Concept of Bioenergetics. Science Press; 2014. [Google Scholar]
- 17. Lei M, Li Y. The advancement on the mitochondrial-associated apoptosis protein. Basic Clin Med. 2012;32(7):837–840. [Google Scholar]
- 18. Dave M, Attur M, Palmer G, et al. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58(9):2786–2797. [DOI] [PubMed] [Google Scholar]
- 19. Yi C, Jiang Y, Shi J, et al. ATP-regulation of antioxidant properties and phenolics in litchi fruit during browning and pathogen infection process. Food Chem. 2010;118(1):42–47. [Google Scholar]
- 20. Leong PK, Leung HY, Chan WM, Ko KM. Differences in the mechanisms by which yang-invigorating and qi-invigorating Chinese tonifying herbs stimulate mitochondrial ATP generation capacity. Chin Med. 2018;9(2):63–74. [Google Scholar]


