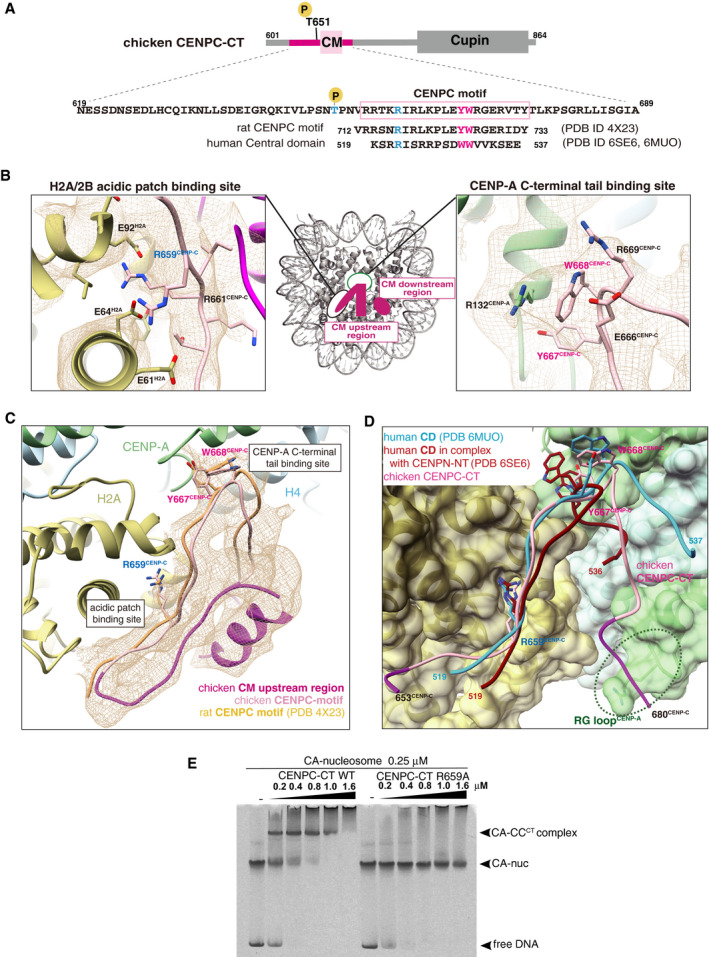
Schematic diagram showing functional elements in chicken CENPC‐CT. The amino acid sequence of CENPC motif (CM) is enclosed in a pink box. The aligned sequences of rat CENPC motif and human central domain (CD), which were used for previous structural studies, are shown at the bottom. Key residues for CENP‐A nucleosome binding, R659, Y667, and W668 in chicken CENP‐C, are colored in blue (R659) and magenta (Y667 and W668). Corresponding residues in rat and human CENP‐C are also colored.
Magnified views of the binding sites for the CENP‐A C‐terminal region and the H2A/2B acidic patch are presented in the cryo‐EM map. Side chains of the key residues are indicated as a stick model.
Cryo‐EM structure of CENPC‐CT bound to the CENP‐A nucleosome. The cryo‐EM density for CENPC‐CT is shown in a mesh representation. The crystal structure of the CENPC motif in complex with the nucleosome (PDB ID: 4X23) is superimposed. The entire backbone structures are well superimposed.
Structural comparison between the chicken CA‐CCCT complex and the human CD structures (PDB ID: 6MUO and 6SE6) on the CENP‐A nucleosome. The structures of CD bound to the CENP‐A nucleosome superimposed to that of the CENPC motif in the CA‐CCCT complex.
CENP‐A nucleosome‐binding assays with WT or R659A mutant of CENPC‐CT. The substitution of R659 residue with alanine (R659A) caused a loss of the CENP‐A nucleosome‐binding ability.