Figure 5. Exclusive CENP‐A nucleosome interaction around the RG loop with CENP‐C and CENP‐N.
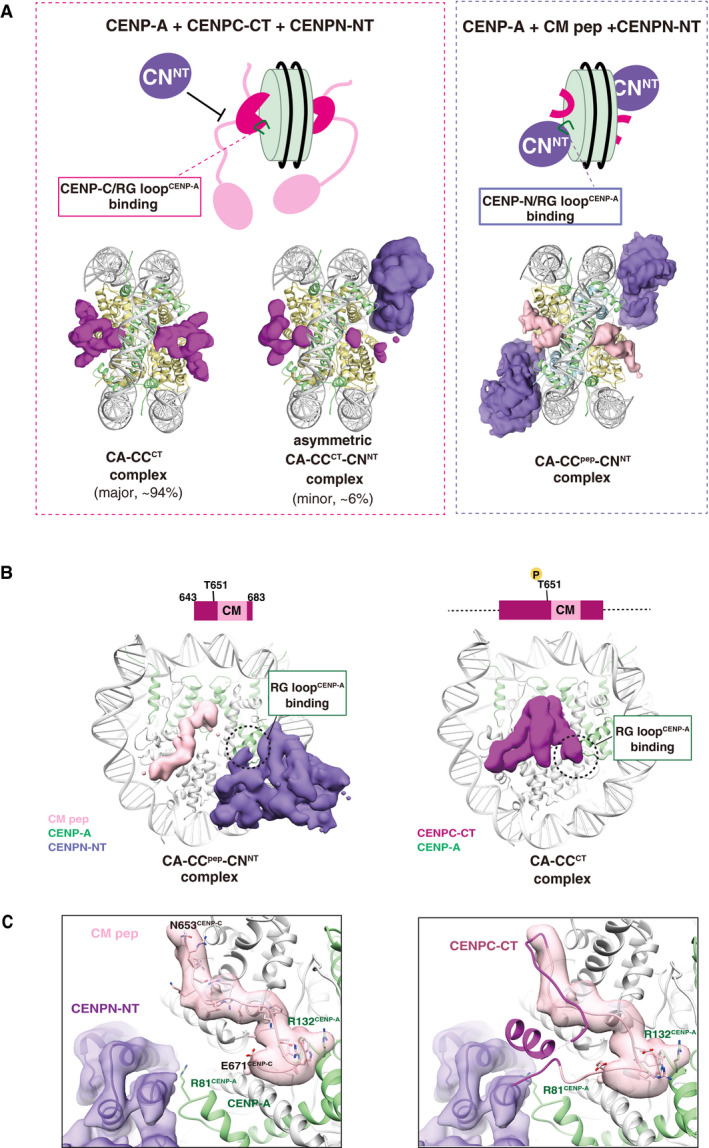
- Structural comparison of the CA‐CCCT with CENPN‐NT (left) and CA‐CCpep with CENPN‐NT (right). The cryo‐EM density maps of the CENPC‐CT fragment, CM peptide, and CENPN‐NT are indicated as a surface representation. The cryo‐EM density maps indicate that the structure of the CENP‐A nucleosomes bound with two CENPC‐CT fragments symmetrically (CA‐CCCT complex) comprised approximately 94% of total analyzed particles. The RG loopCENP‐A is occupied by CENPC‐CT in the CA‐CCCT complex and thereby the CENPN‐NT could not access the same side of the nucleosome. The minor fraction (approximately 6% of total analyzed particles) comprised asymmetric CA‐CCCT‐CNNT complexes. In the structures of the CA‐CCpep‐CNNT (right), CM peptide and CENPN‐NT simultaneously bind to the CENP‐A nucleosome.
- Side views of the CA‐CCpep‐CNNT and CA‐CCCT complexes. The RG loopCENP‐A binding site in each complex is indicated by a dotted circle.
- The left panel contains the structure of the RG loopCENP‐A binding site in the CA‐CCpep‐CNNT complex. R81CENP‐A of the RG loop is recognized by CENPN‐NT. The right panel displays a superposition of the structure of CENPC‐CT in the CA‐CCCT complex on the CA‐CCpep‐CNNT complex shown in the left panel. The model of CM peptide in the CA‐CCpep‐CNNT complex has been removed for clarity. The CENPC motif region in CENPC‐CT is shown in pink. Structural collision between the CM downstream region of CENPC‐CT and CENPN‐NT is shown at RG loopCENP‐A in the CENP‐A nucleosome.
