Figure 6. A model of the dynamic change of binding partners of the CENP‐A nucleosome during the cell cycle progression.
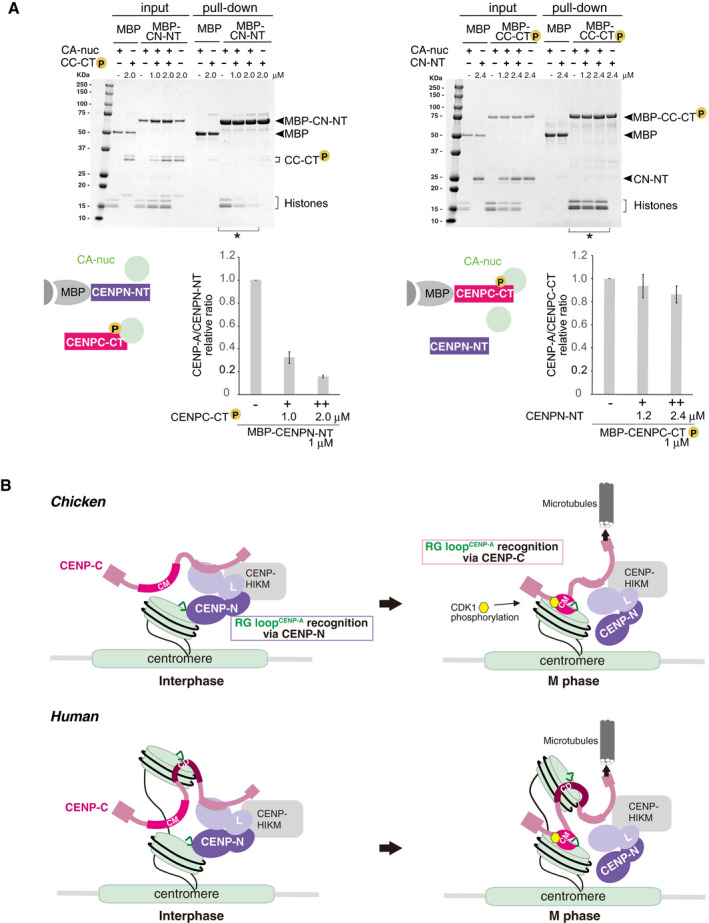
- Competitive pull‐down assays for the CENP‐A nucleosome binding of CENPC‐CT and CENPN‐NT. The left panel displays a complex of MBP‐CENPN‐NT (MBP‐CN‐NT) with the CENP‐A nucleosome (CA‐nuc) incubated in the absence or presence of phosphorylated CENPC‐CT (CC‐CT) and pulled down by MBP affinity. The right panel displays a complex of phosphorylated MBP‐CENPC‐CT (MBP‐CC‐CT) with the CENP‐A nucleosome (CA‐nuc) incubated in the absence or presence of CENPN‐NT (CN‐NT) and pulled down by MBP affinity. The signal intensities of all histones in CENP‐A nucleosomes, which were precipitated with MBP proteins in indicated lanes (*), were quantified. The signal intensities were normalized to MBP‐CN‐NT or MBP‐CC‐CT signals. Bar graph indicates mean with SD (n = 3).
- A model of the dynamic change of binding partners of the CENP‐A nucleosome during the cell cycle progression in chicken (upper) or human (bottom). During the interphase, chicken CENP‐C without phosphorylation at T651 is dissociated from the CENP‐A nucleosome. Then, CENP‐N can bind to the RG loopCENP‐A of the CENP‐A nucleosome in interphase cells. In mitosis, the CENP‐C C‐terminal region is phosphorylated by CDK1, and this phosphorylation facilitates CENP‐A‐CENP‐C interaction. The stable CENP‐A‐CENP‐C interaction excludes CENP‐N from the RG loop. This phospho‐regulation by the CENP‐C C‐terminal region is conserved in chicken and human. In humans, the second CENP‐A binding region (central domain, CD) might associate with the CENP‐A nucleosome throughout the cell cycle. However, it is still unclear how CD is involved in formation of centromeric chromatin structure (bottom).
