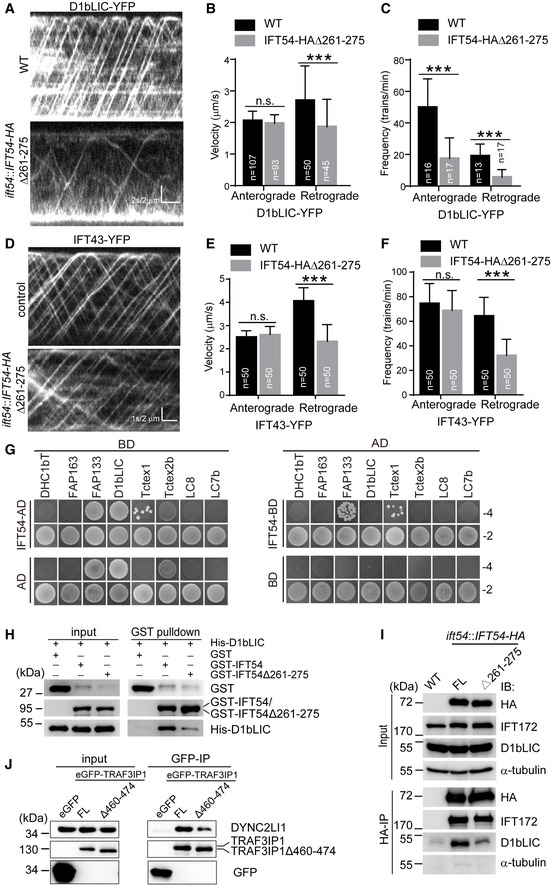
Figure 5. IFT54 interacts with IFT dynein subunit D1bLIC and deletion of residues 261–275 of IFT54 disrupts this interaction and impairs anterograde IFT of D1bLIC.
-
AKymograms showing the trajectories of IFT trains inside cilia as visualized with D1bLIC‐YFP. Wild‐type (WT) and IFT54Δ261–275 mutant cells expressing D1bLIC‐YFP were analyzed by live imaging via TIRF microscopy.
-
B, CVelocities (B) and frequencies (C) of anterograde and retrograde IFT of D1bLIC‐YFP. n represents the number of cilia assayed from four independent experiments. Values show the mean ± SD. Unpaired two‐tailed Student’s t‐test analysis, n.s.: no significance; ***P < 0.0001.
-
DKymograms of IFT43‐YFP of WT and mutant cilia.
-
E, FVelocities (E) and frequencies (F) of anterograde and retrograde IFT of IFT43‐YFP. n represents the number of cilia assayed from three independent experiments. Values show the mean ± SD. Unpaired two‐tailed Student’s t‐test analysis, n.s.: no significance; ***P < 0.0001.
-
GYeast two‐hybrid assay for interaction between IFT54 and IFT dynein subunits. Yeast cells that were transformed with each pair of constructs as indicated were grown under selection media lacking leucine, tryptophan, histidine, and adenine (−4) or lacking leucine and tryptophan (−2). DHC1bT, DHC1b tail domain; Empty AD or BD vectors were used as control.
-
HInteraction of IFT54 and IFT54Δ261–275 with D1bLIC by GST pull‐down assay. Bacterial expressed GST, GST‐IFT54, or GST‐IFT54Δ261–275 were mixed, respectively, with His‐D1bLIC followed by GST pull‐down and immunoblotting with anti‐GST and anti‐His antibodies. The normalized ratio of His‐D1bLIC in the pull‐down samples (GST‐IFT54 versus mutant) is 1:0.32.
-
ICo‐immunoprecipitation of IFT54 and IFT54Δ261–275 with D1bLIC. Cell extracts from wild‐type (WT), ift54 expressing HA‐tagged full‐length (FL) IFT54 or IFT54Δ261–275 mutant were subjected to immunoprecipitation with anti‐HA antibody followed by immunoblotting with the indicated antibodies. Please note that IFT54Δ261–275 mutant exhibited weaker interaction with D1bLIC relative to the control. The normalized ratio of D1bLIC in the immunoprecipitates (IFT54‐HA versus mutant) is 1:0.37.
-
JInteraction of IFT54 with D1bLIC is conserved in mammalian cells. Cell extracts from HEK293T cells expressing eGFP, eGFP‐tagged TRAF3IP1, or TRAF3IP1Δ460–474 (corresponding deletion mutant of IFT54Δ261–275) were analyzed by immunoprecipitation with GFP antibody followed by immunoblotting with anti‐GFP and DYNC2LI1, human homologue of D1bLIC, antibodies, respectively. Please note that the deletion mutant weakened the interaction with IFT dynein subunit DYNC2LI1 relative to the control. The normalized ratio of DYNC2LI1 in the immunoprecipitates (TRAF3IP1 versus mutant) is 1:0.49.
Source data are available online for this figure.
