Figure 1. Intrinsic and extrinsic apoptosis.
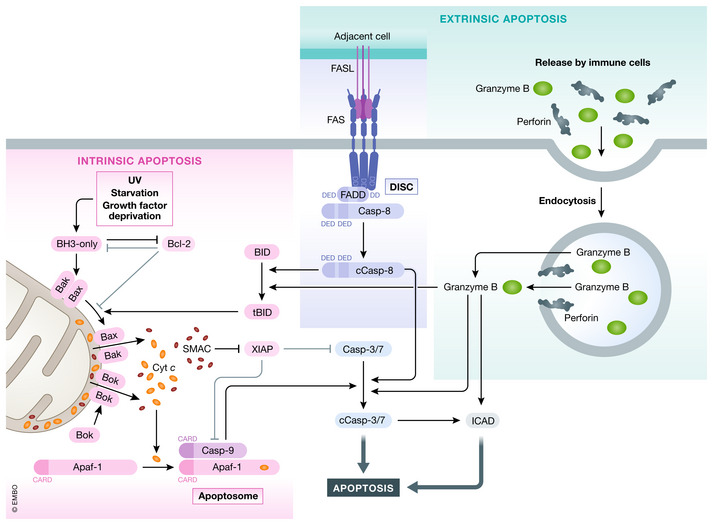
Intrinsic apoptosis can be induced by various stimuli (e.g., GF (growth factor) withdrawal) by shifting the equilibrium of pro‐survival Bcl‐2 and BH3‐only proteins. Sequestering of pro‐survival Bcl‐2 proteins or directed binding of BH3‐only proteins to Bax and Bak induces oligomerization of Bax and Bak leading to MOMP and cytochrome c and Smac release. Bok can lead to MOMP independent of Bcl‐2 family members. Released cytochrome c binds Apaf‐1 and induces apoptosome formation which recruits caspase‐9. Activated caspase‐9 induces caspase‐3/7 cleavage and activation (cCasp3/7) leading to apoptosis. Extrinsic apoptosis can be induced by binding of select group of TNF family ligands to, their receptors leading to DISC formation by recruitment of adapter FADD/TRADD and caspase‐8. Caspase‐8 autoprocesses itself (cCasp8—cleaved/activated caspase‐8) and can directly activate caspase‐3 or cleave Bid to generated tBid and triggers intrinsic apoptosis. Caspase‐3/7/9 activity can be inhibited by XIAP, which itself can be antagonized by Smac. Lastly, apoptosis can also be induced by granzyme B and Perforin released form immune cells. Once taken up by the target cell, granzyme B can induces cell death by caspase‐3 or by directly activating apoptosis effectors (e.g., CAD).
