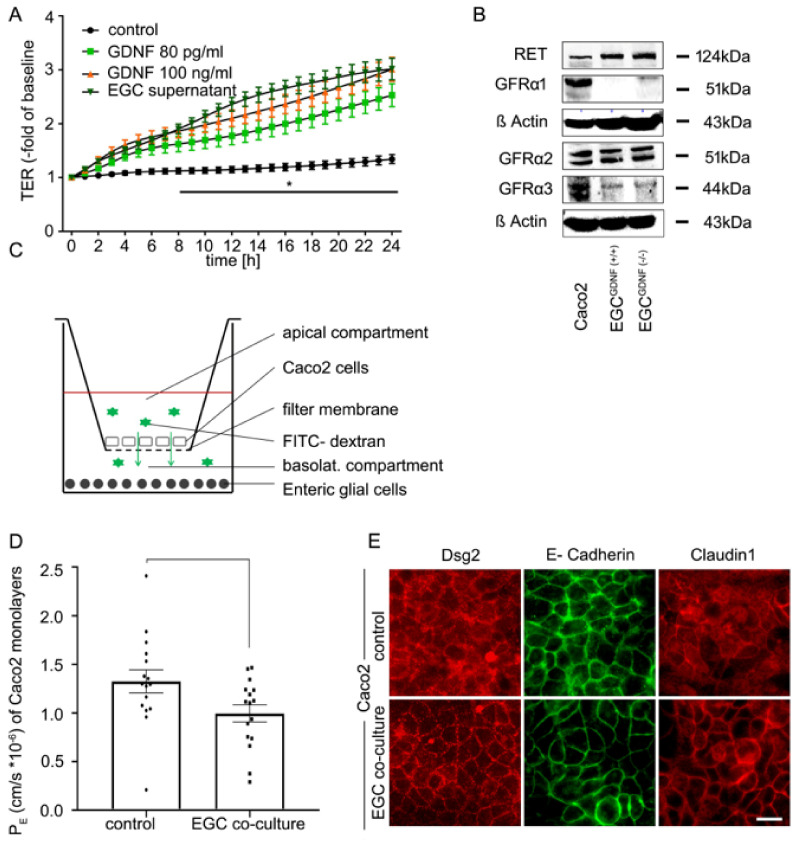
Figure 2.
Co-culture of enteric glial cells (EGC) with Caco2 cells stabilizes epithelial barrier function. (A). Measurements of transepithelial electric resistance (TER) showed a significant increase beginning at 8 h after incubation with the supernatant of EGC and raised to 3.01 ± 0.1-fold of baseline (equates to 2.24-fold of controls) while incubation with glial cell line-derived neurotrophic factor (GDNF) at 100 ng/mL increased TER significantly to 2.99 ± 0.07 of baseline (2.23-fold of controls) after 24 h. Similar results were obtained when GDNF was applied at 80 pg/mL when TER increased to 2.52 ± 0.09 -fold of baseline (1.88-fold of controls); (n = 6, * = significant different vs. control after 8 h for all other conditions; p < 0.05, two-way ANOVA). Control = Caco2 monolayer without treatment. (B). Western Blots of Caco2 cells and EGC cells (wilde type (WT) and GDNF-knockdown) confirmed that all these cell lines express GDNF receptors and RET (n = 3). Quantification of the blots is shown in Supplementary Figure S1 (C). Schematic picture of the co-culture model of Caco2 cells and enteric glia cells. (D). Permeability coefficients (PE) of 4-kDa FITC dextran flux across Caco2 monolayers were significantly reduced 24 h after co-culture with EGC (p < 0.05, n = 16, student’s t-test). Control = Caco2 monolayer without treatment. (E). Immunostaining of the transwell filters showed an augmented and more linear staining pattern of desmosomal protein Desmoglein2 (Dsg2), adherens junction protein E-Cadherin and tight junction protein Claudin1 24 h after co-culture of confluent Caco2 cells with EGC compared to untreated controls (representatives are shown for n = 10, scale = 20 µm). Control = Caco2 monolayer without treatment.