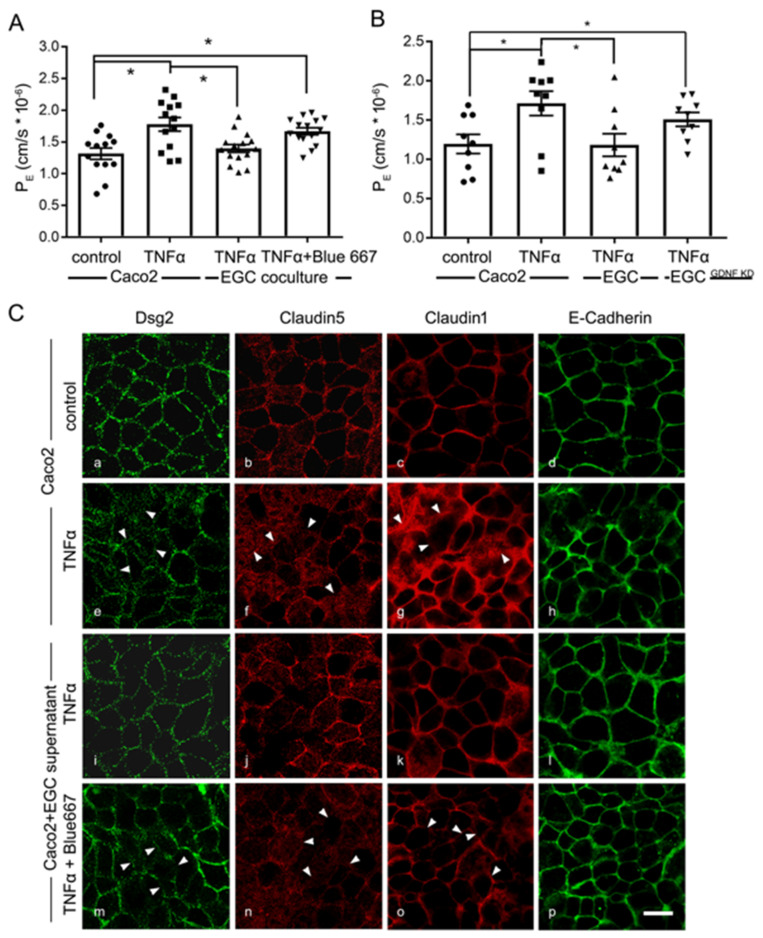
Figure 8.
Barrier protective effects by enteric glial cells (EGC) in inflammation are glial cell line-derived neurotrophic factor (GDNF)-dependent. (A). Permeability coefficients (PE) of 4-kDa FITC dextran flux across Caco2 monolayers was increased following the application of Tumor necrosis factor α (TNFα) to 1.78 ± 0.10 × 10−6 compared to 1.32 ± 0.08 × 10−6. This was blocked under co-culture conditions together with EGC cells. Protective effects of the co-culture were diminished by Blue667 when PE was 1.67 ± 0.06 × 10−6 (n = 9; * = p < 0.05; One-way ANOVA). Control = Caco2 monolayer without treatment. (B). In PE measurements barrier protection of EGCs after TNFα incubation were absent when co-cultures were performed with EGCGDNF KD (n = 9; * = p < 0.05; One-way ANOVA). (C). Representative immunostaining for junctional proteins Dsg2, E-Cadherin, Claudin1 and 5 are shown. TNFα reduced the staining pattern of these junctional proteins at the cell borders of Caco2 cells. This effect was attenuated when Caco2 cells were cultivated with EGC supernatants. The beneficial effects of EGC supernatants were blocked by RET Inhibitor Blue667; arrowheads point to examples of reduced or lost staining patterns at the cell borders (representatives are shown for n = 6, Scale 20 µm); Control = Caco2 monolayer without treatment.