Table 1.
Akt inhibitors in the management of HCC.
| Inhibitor | Mechanism of Action, Structure | Experiment Setup | Antitumor Effect | Effect on TME | Clinical Trial |
|---|---|---|---|---|---|
| GDC-0068 [64,65,66] |
ATP competitive AKT inhibitor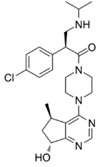
|
Combination of GDC-0068 with Sorafenib in HepG2 and Huh7 sorafenib-resistant cell-lines |
|
Not investigated | Phase-I/II including multiple solid tumors treated by GDC-0068 in monotherapy or in association with abiraterone + prednisolone: safe and tolerable in monotherapy or in combination |
| AZD5363 [67,68] |
ATP competitive AKT inhibitor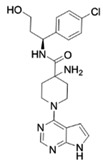
|
Single agent in HepG2 and Huh-7 HCC cells Combination with FH535 (β-catenin inhibitor) in THH, Hep3B and HepG2 |
|
Not investigated | Phase-I, AZD5363 in monotherapy, in multiple advanced solid tumors including liver cancer (NCT01895946): safe and tolerable, no data on antitumor response Phase-I including multiple solid tumors harboring AKT mutations (NCT02465060) |
| ARQ 092 [69,70] |
allosteric pan-AKT inhibitor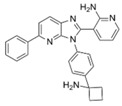
|
Single agent and in combination with Sorafenib in a DEN-induced cirrhotic rat model of HCC and in Hep3B, HepG2, Huh-7, PLC/PRF, and HR4 HCC cell lines |
|
|
No clinical trial |
| ARQ 751 [71] |
allosteric pan-AKT inhibitor Chemical structure of ARQ 751 is currently unavailable. |
Single agent and in combination with sorafinib in DEN-induced cirrhotic rat model of HCC |
|
|
Phase-Ib (NCT02761694) in solid tumors with PIK3CA/AKT/PTEN mutations including HCC: ongoing |
| MK-2206 [72,73] |
allosteric pan-AKT inhibitor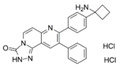
|
Single agent in Human Huh7, Hep3B, and HepG2 HCC cell lines |
|
Not investigated | Phase-II trial, MK-2206 in monotherapy in advanced HCC previously treated (NCT01239355): discontinued due to discouraging results |
