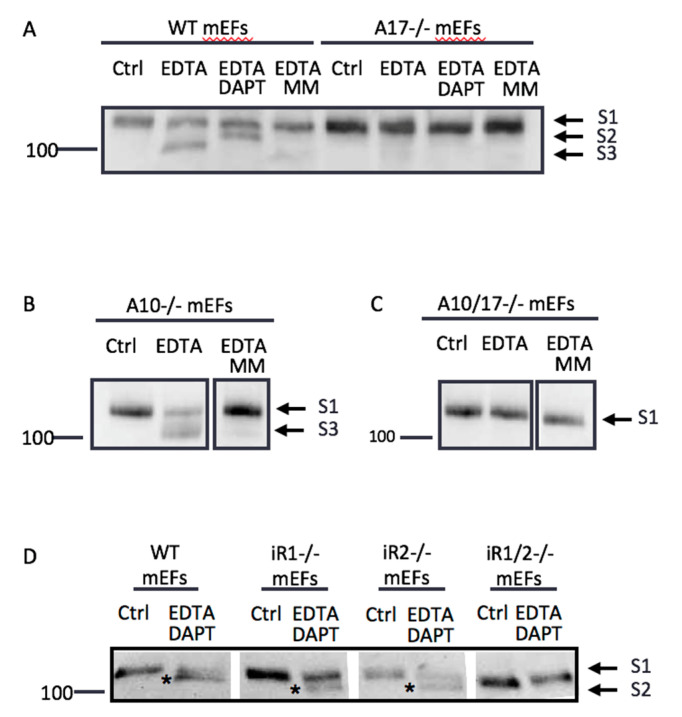
Figure 2.
ADAM17 is the primary S2 protease involved in EDTA-induced endogenous Notch1 processing. Cells were treated with PBS (vehicle control, Ctrl), 5 mM of EDTA, or 5 mM of EDTA in the presence of the inhibitors DAPT (5 µM) or marimastat (5 µM MM, metalloprotease inhibitor that blocks ADAMs10 and 17) for 15 min. MM blocks the S2 cleavage, whereas DAPT blocks S3 processing. All the inhibitors were pre-incubated for 2 h in Opti-MEM and Western blots were performed using an anti-Notch1 cytoplasmic domain antibody. (A) Samples of WT mEFs treated with 5 mM of EDTA showed a band around the expected size of S3, which was not produced in the presence of DAPT. Instead, a fragment around the expected size of the Notch1 S2 product accumulated upon DAPT treatment. WT samples treated with EDTA and MM showed no S2 and S3 products. In contrast, mEFs lacking ADAM17 (A17-/- mEFs) showed no S2 or S3 products under the conditions where these bands were observed for WT mEFs. (B) ADAM10-deficient mEFs (A10-/- mEFs) showed the accumulation of an S3 product after EDTA treatment, which was blocked by MM, just like in WT mEFs. (C) Double-knockout mEFs deficient in ADAM10 and ADAM17 (A10/17-/- mEFs) resembled A17-/- mEFs in that they showed no S3 production in response to EDTA treatment. (D) mEFs deficient in the ADAM17 regulators iRhoms 1 and 2 (iR1/2-/- mEFs) also produced no S2 fragment in the presence of EDTA and DAPT, whereas the inactivation of either iRhom1 or iRhom2 (iR1-/- or iR2-/- mEFs) did not prevent the generation of Notch1 S2 (indicated by an asterisk). All Western blots shown are representative of at least 3 independent experiments.