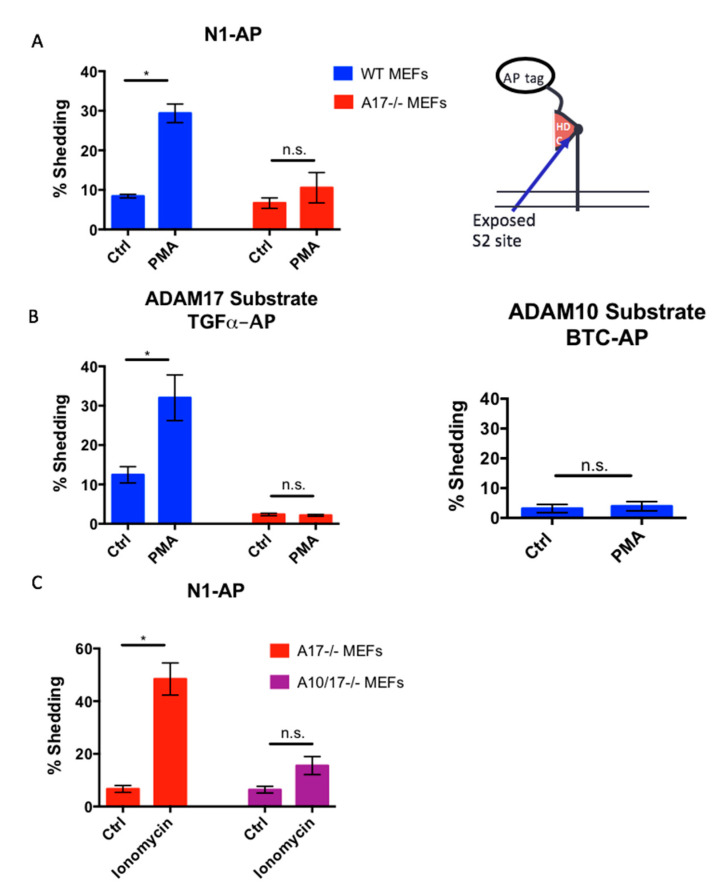
Figure 5.
The constitutively exposed Notch1 S2 site behaves like an ADAM17 substrate. (A) WT and A17-/- mEFs were transfected with a truncated Notch1 receptor, consisting of the C-terminal portion of the receptor starting from amino acid residue 1687 within the C-terminal part of the heterodimerization domain, with an alkaline phosphatase tag attached to its N-terminus (Notch1-AP or N1-AP), and were stimulated with the ADAM17-selective stimulus PMA [52]. WT mEFs stimulated with 25 ng/mL of PMA had increased shedding of N1-AP over untreated WT mEFs. There was no significant increase in the PMA-induced shedding of N1-AP in A17-/- mEFs. (B) N1-AP shedding thus behaved similarly to the shedding of TGFα-AP, a known ADAM17 substrate [35,52], which could be stimulated by PMA in WT mEFs, but not in A17-/- mEFs. In contrast, PMA treatment did not result in the shedding of an established ADAM10 substrate, betacellulin-AP (BTC-AP) [52]. (C) In A17-/- mEFs treated with 2.5 µM of ionomycin, which strongly stimulates ADAM10 and ADAM17, N1-AP shedding was increased over unstimulated mEFs, whereas there was no statistically significant increase in N1-AP shedding in A10/17-/- mEFs treated with ionomycin, suggesting that ADAM10 can shed N1-AP in the absence of ADAM17. All the data are shown as mean ± standard error of the mean (SEM) for n ≥ 3 independent experiments. An * indicates p < 0.05 and n.s. indicates no statistically significant difference using the unpaired two-tailed Student’s t-test.