Table 1.
In vitro activity of compounds 1a,b–6a,b.
| Structure | Series A R = m-CNPh |
Series B R = p-NO2Ph |
|||
|---|---|---|---|---|---|
| %RA * | IC50 ** (µM) | %RA * | IC50 ** (µM) | ||
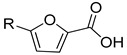
|
I | II | |||
| 3.1 ± 1.0 | 6.3 ± 0.9 | 18.2 ± 5.1 | 7.6 ± 1.6 | ||
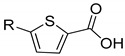
|
1a | 1b | |||
| 23.7 ± 5.0 | 35.5 ± 1.9 | 22.5 ±10.8 | 18.6 ± 1.7 | ||
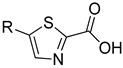
|
2a | 2b | |||
| 24.8 ± 1.8 | 41.9 ± 7.3 | 32.8 ± 2.3 | - | ||
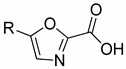
|
3a | 3b | |||
| 38.0 ± 7.6 | - | 23.0 ± 7.8 | 21.1 ± 2.7 | ||

|
4a | 4b | |||
| 21.2 ± 1.0 | 39.3 ± 3.0 | 18.2 ± 4.9 | 27.5 ± 2.3 | ||
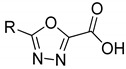
|
5a | 5b | |||
| 75.0 ± 9.8 | - | 59.0 ± 4.3 | - | ||
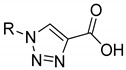
|
6a | 6b | |||
| 67.9 ± 6.6 | - | 32.7 ± 7.8 | - | ||
* % residual enzymatic activity at 100 µM; ** only for compounds with %RA ≤ 25%.
