Abstract
Clostridioides difficile is an anaerobic pathogen that causes significant morbidity and mortality. Understanding the mechanisms of colonization resistance against C. difficile is important for elucidating the mechanisms by which C. difficile is able to colonize the gut after antibiotics. Commensal Clostridium play a key role in colonization resistance. They are able to modify bile acids which alter the C. difficile life cycle. Commensal Clostridium also produce other inhibitory metabolites including antimicrobials and short chain fatty acids. They also compete with C. difficile for vital nutrients such as proline. Understanding the mechanistic effects that these metabolites have on C. difficile and other gut pathogens is important for the development of new therapeutics against C. difficile infection (CDI), which are urgently needed.
Keywords: Clostridioides difficile, Clostridium scindens, secondary bile acids, deconjugation, dehydroxylation, epimerization, short-chain fatty acids, proline, hydroxyproline
1. Introduction
Clostridioides difficile is an anaerobic, spore-forming, toxigenic bacterial pathogen that was first isolated from the stool of newborn infants in 1935 [1]. C. difficile infection (CDI) is the cause of significant morbidity and mortality and is responsible for over 4.8 billion dollars in excess medical costs yearly [2,3]. While the current first line treatment of vancomycin is capable of resolving CDI, 20–30% of patients will experience a recurrence within 30 days. Additionally, 40–60% of patients who experience recurrent CDI once will have multiple recurrences [4,5]. The use of antibiotics, including vancomycin, is a significant risk factor for CDI due to its ability to alter the gut microbiota, resulting in a loss of colonization resistance against C. difficile [6,7,8]. Colonization resistance is defined as the ability of the indigenous gut microbiota to protect against colonization by pathogens such as C. difficile [9]. Understanding the mechanisms of colonization resistance against C. difficile is important for determining the mechanisms by which C. difficile is able to colonize the gut after antibiotics and is important for developing new therapeutics and preventatives for CDI. While there are different mechanisms of colonization resistance, there is evidence that commensal gut bacteria from the genus Clostridium may play a key role, especially those capable of producing secondary bile acids, which are inhibitory to C. difficile [10,11,12,13,14]. In this review, we highlight how commensal Clostridium found in the gut are able to alter colonization resistance against C. difficile, with a particular emphasis on the production of secondary bile acids and other inhibitory metabolites, as well as competition for nutrients.
2. Primary and Secondary Bile Acids Alter the C. Difficile Life Cycle
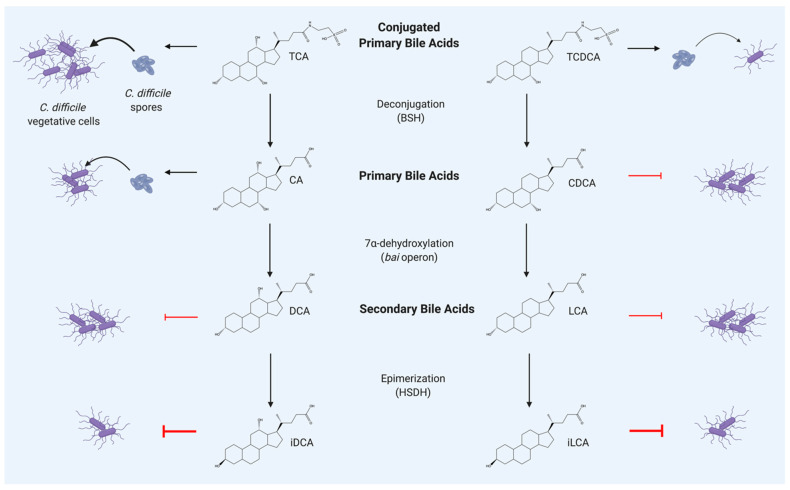
Bile acids are important signaling molecules that modulate various metabolic functions, play an essential role in fat digestion, and help shape the gut microbiota [15,16]. Primary, or host-derived, bile acids are synthesized in the liver from cholesterol in a multistep enzymatic process via the classical or alternative pathway [17,18]. The classical pathway generates cholate (CA) and chenodeoxycholate (CDCA), whereas the alternative pathway predominately synthesizes CDCA [18]. Primary bile acids, as well as secondary bile acids, that have gone through enterohepatic circulation are conjugated with either taurine or glycine, which makes them impermeable to cell membranes, permitting higher concentrations of bile acids within bile and the gut [19]. These conjugated bile acids are released into the duodenum in response to food ingestion [18,20]. In the small intestine, conjugated primary bile acids are deconjugated by bile salt hydrolases (BSHs) commonly encoded by gut bacteria [21]. After deconjugation, these primary bile acids are further altered by bacteria in the colon in a myriad of ways to create a diverse pool of secondary, or microbiota-derived bile acids. Common secondary bile acids found in the gut include deoxycholate (DCA) and lithocholate (LCA), which are generated by 7α-dehydroxylation from CA and CDCA, respectively [21,22]. DCA and LCA can be epimerized by hydroxysteroid dehydrogenases (HDSHs), generating such bile acids as ursodeoxycholate (UDCA), iso-DCA (iDCA), and iso-LCA (iLCA) [23].
Primary and secondary bile acids significantly alter the C. difficile life cycle. While bile acids are known to have detergent-like properties that can disrupt bacterial cellular membranes and cause cell lysis, they also affect spore germination, outgrowth, and toxin activity of C. difficile in vitro at sub-inhibitory concentrations [24,25,26]. In particular, taurocholate (TCA) is a powerful germinant for C. difficile spores, as shown in Figure 1 [25]. Primary bile acids glycocholate (GCA) and CA and the secondary bile acid DCA also stimulate spore germination, while the primary bile acid CDCA, and the secondary bile acids LCA and UCDA inhibit germination of C. difficile spores in vitro [24,25,27,28]. Secondary bile acids hyodeoxycholate (HDCA), DCA, iDCA, UDCA, LCA, and iLCA decrease the growth of C. difficile in vitro in a dose dependent manner, as shown in Figure 1, and also reduce toxin activity in some strains of C. difficile [24,25,29,30]. While the mechanism of how these bile acids alter C. difficile has yet to be fully defined, some progress has been made using proteomic approaches. Specifically, when actively growing C. difficile is exposed to sub-inhibitory concentrations of CA, DCA, CDCA, or LCA in vitro, the abundance of cell wall binding proteins, cellular chaperones, and cell division proteins increase [26]. When C. difficile is grown with sub-inhibitory concentrations of CA, DCA, CDCA, or LCA for a longer period of time, the abundance of alcohol dehydrogenases AdhE1 and AdhE2 decrease, inhibiting the conversion of acetyl-CoA to butynol or ethanol [26]. This indicates that bile acid stress alters the flux through central metabolic pathways of C. difficile as well as causing more generalized stress responses. Bile acids also affect enzymes required for Stickland fermentation, which is required for the growth of C. difficile and several other bacteria in the genus Clostridium [27,28]. Stickland fermentation allows amino acids to be used as an energy source by coupling the oxidation and reduction of paired amino acids to the formation of ATP [29]. Most of the enzymes involved in the reductive Stickland fermentation of leucine to isocaproate increase in abundance when cells are exposed to CA, DCA, CDCA, or LCA [26]. The addition of CA or DCA causes an increased abundance of the proline reductase enzymes PrdA, PrdB, and PrdC, which are required for Stickland fermentation of proline in C. difficile, while the addition of CDCA or LCA causes a decreased abundance of those same three enzymes. This indicates that different bile acids can alter C. difficile metabolism. Further studies are needed to clarify how specific bile acids are able to shape the formation and activity of proline reductase enzymes, as well as the effect that the altered expression of Stickland fermentation enzymes has on the competitive fitness of C. difficile.
Figure 1.
Selected transformations of bile acids carried out by gut bacteria and their effect on Clostridioides difficile. TCA is a strong germinant for C. difficile spores and TCDCA is a weak germinant. CA is a moderate germinant for C. difficile spores, and CDCA inhibits vegetative C. difficile. The secondary bile acids DCA and LCA inhibit vegetative C. difficile and iDCA and iLCA strongly inhibit C. difficile. Abb. BSH, bile salt hydrolase; bai, bile acid inducible; HSDH, hydroxysteroid dehydrogenase; TCA, taurocholate; TCDCA, taurochenodeoxycholate; CA, cholate; CDCA, chenodeoxycholate; DCA, deoxycholate; LCA, lithocholate; iDCA, isodeoxycholate; iLCA, isolithocholate. Created with BioRender.com.
Select bile acids can also induce morphological changes in C. difficile cells. CDCA, DCA, and LCA cause a significant decrease in the presence of flagella, as well as the flagellar structural protein FliC, and flagellar filaments disappear almost entirely when C. difficile is challenged with LCA in vitro [26]. In addition, bacterial cells challenged with CA, DCA, or CDCA were significantly longer than the untreated cells, which is an indicator of bacterial stress, but the addition of LCA does not affect cell shape [26]. DCA causes a significant increase in biofilm formation by C. difficile in vitro, whereas LCA does not impact biofilm formation [30]. While CDCA, DCA, and LCA are able to impact toxin activity in C. difficile in vitro, the mechanism was unknown until recently [24,31]. Bile acids, including DCA and LCA, bind in a reversible fashion to TcdB, one of the two primary toxins carried by C. difficile [31]. LCA and CDCA are able to bind to TcdB with high efficiency and they are able to inhibit cell rounding, a sign of cell death, in human fibroblast cells [31]. DCA binds to TcdB with lower efficiency than LCA and CDCA and does not inhibit cell rounding in human fibroblasts [31]. This binding induced a major conformational change in TcdB, which inhibited the ability of the toxin to bind cell surface receptors of HCT 116 cells, a human colonic cell line [31]. This mechanistic in vitro work demonstrates that bile acids elicit dynamic effects on C. difficile and manipulation of the bile acid pool could be a promising therapeutic strategy for treating CDI.
Secondary bile acids are also associated with protection against CDI in mouse models and human subjects [7,10,32,33,34,35,36]. An increase in primary bile acids and a loss of secondary bile acids is observed after treatment with antibiotics and is associated with increased susceptibility to CDI [7,8,32,33,34,35,36]. Cecal extracts from mice made susceptible to CDI stimulate C. difficile spore germination, while cecal extracts from mice resistant to CDI inhibit spore germination, indicating that antibiotic-induced changes in bile acid levels in vivo are sufficient to induce germination and outgrowth of C. difficile spores [36,37]. However, C. difficile spores are able germinate in the small intestine prior to antibiotics, indicating that the bile acids present in the small intestine do not protect against CDI [37]. After human fecal microbiota transplantation (FMT), an increase in microbial diversity is observed and secondary bile acid metabolism is restored [38,39]. Specifically, the levels of secondary bile acids including DCA, LCA, and UCDA are increased and the primary bile acids CA and CDCA are decreased in CDI patients after receiving an FMT [39]. In addition, fecal samples of patients with CDI have a lower prevalence of baiCD, a gene present in commensal Clostridium required for the synthesis of DCA and LCA via 7α-dehydroxylation, although baiCD has also been found in the stool samples of individuals with failed FMTs [12,40]. Clostridium scindens is a commensal bacterium found in the gut microbiota [41]. It produces DCA and LCA and is associated with the return of colonization resistance against C. difficile in a mouse model of CDI, however C. scindens has also been found to be present in the stool samples of individuals with CDI [10,42]. Mice that receive C. scindens before being challenged with C. difficile show increased levels of LCA, although levels of most other bile acids are unchanged [10]. While manipulation of the bile acid pool using commensal bacteria is a promising strategy, the addition of exogenous bile acids can also affect the progress of CDI. Challenging mice exogenously with the secondary bile acid UDCA attenuates disease early during CDI, and also alters the fecal bile acid metabolome without significantly altering the gut microbiome [43].
3. Bile Acid Altering Enzymes Encoded by Commensal Clostridium
3.1. Bile Salt Hydrolases
Bile salt hydrolases (BSHs) are microbial enzymes that deconjugate primary and secondary bile acid from the amino acids they are conjugated to, usually taurine and glycine [44]. While BSHs are commonly encoded by multiple members of the gut microbiota, commensals in the genus Clostridium rarely encode BSHs, although Clostridium hiranonis and the pathogen Clostridium perfringens both encode BSHs and have demonstrated BSH activity [21,45]. C. hiranonis is the only bacterium to date known to have the capability for both 7α-dehydroxylation and deconjugation [45]. The presence of BSHs in the gut are hypothesized to be important for several reasons. BSHs are considered the gateway step for the transformation of primary bile acids to secondary bile acids, as further transformations cannot occur until the conjugated amino acid is removed [21]. The taurine or glycine that is released when deconjugation occurs may be acquired for nutrition by members of the gut microbiota [46]. A recent study showed that bile acids can also be conjugated with tyrosine, phenylalanine, or leucine in mice, however deconjugation of these conjugated bile acids by BSHs is unknown at this time [47]. Interestingly, one strain of an unnamed Clostridium bacterium capable of deconjugation shows increased growth when taurine was added to the growth medium, indicating that BSH activity might be nutritionally beneficial [48]. Taurine is also enriched in the feces of pediatric inflammatory bowel disease patients with CDI, indicating a potential association between C. difficile and taurine [49].
In addition, a bsh encoded by Bifidobacterium longum is transcriptionally coupled to glnE (glutamine synthetase adenylyltransferase), which indicates that deconjugation activity may be coupled to nitrogen regulation [50]. However, lactobacilli grown with conjugated bile acids do not utilize the steroid moiety of the bile acid for cellular precursors and taurine does not affect growth, indicating that not all bacteria encoding a bsh obtain a direct nutritional benefit from deconjugation [51]. BSH activity has been hypothesized to detoxify conjugated bile acids by converting them to a less toxic form, as the bsh encoded by Listeria monocytogenes is important for resistance to bile in vitro and is an important virulence factor in animal models [52]. However, unconjugated bile acids are more toxic to some Lactobacillus spp. than their conjugated forms, meaning that deconjugation of bile acids can cause an increase in toxicity for at least some members of the gut microbiota [53,54]. Conjugated bile acids are more soluble than deconjugated bile acids, so the increased toxicity observed may be offset by the decreased bioavailability that occurs when micelles form [55,56]. In addition, deconjugation is important for producing free bile acids available for 7α-dehydroxylation [41,57]. BSH activity is also correlated with resistance to C. difficile after FMT [58]. Pre-FMT stool samples harbor reduced BSH activity and a lower proportion of BSH-producing bacterial species when compared with donor stool and post-FMT stool. Additionally, mice inoculated with Escherichia coli expressing a highly active BSH have a ~70% reduction in C. difficile viable counts when compared to mice inoculated with non-BSH expressing E. coli [58]. This indicates that BSH activity could be a significant contributor to the efficacy of FMT in treating recurrent CDI.
3.2. Bile Acid Inducible Operon
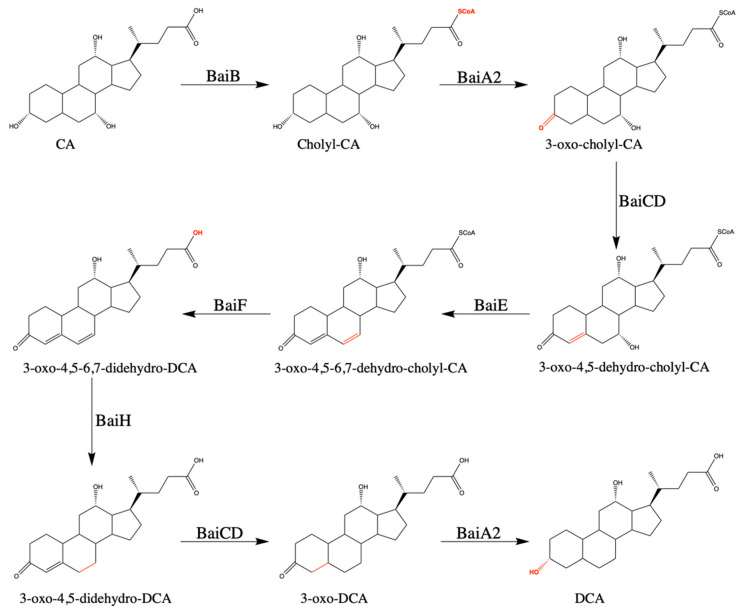
Commensal Clostridium that harbor the bile acid inducible (bai) operon are capable of synthesizing DCA from CA and LCA from CDCA via 7α-dehydroxylation. While the enzymes responsible for the steps in the oxidative arm of the metabolic pathway have been known for some time, the reductive arm has only recently been defined by reconstructing the pathway in vitro [59]. The proton-dependent transporter BaiG is responsible for transporting the primary unconjugated bile acid into the cell [60]. Six core enzymes encoded by the bai operon are sufficient for completing the 7α-dehydroxylation pathway, as shown in Figure 2 [59]. Coenzyme A is ligated onto the substrate in an ATP dependent manner by BaiB and the dehydrogenase BaiA2 oxidizes the 3-hydroxy group [61,62]. The NADH/flavin-dependent oxidoreductase BaiCD catalyzes the formation of the C4=C5 bond and the 7α-dehydratase BaiE catalyzes the formation of the C6=C7 bond by removing the 7α hydroxyl group [12,63]. The 7α-dehydration is the last step in the oxidative arm of the pathway and is irreversible and rate limiting [64]. The reductive arm of the 7α-dehydroxylation consists of four steps. The removal of Coenzyme A is catalyzed by the bile acid-CoA hydrolase BaiF, which can also ligate CoA onto the primary unconjugated bile acid in an ATP independent manner [65,66]. The NADH/flavin-dependent oxidoreductases BaiH and BaiCD catalyze the removal of the C6=C7 and C4=C5 bonds and BaiA2 performs the final reductive step, catalyzing the transformation from 3-oxo-DCA to DCA [59]. The enzyme responsible for transport of DCA out of the cell has yet to be determined.
Figure 2.
Proposed pathway for conversion from CA to DCA via 7α-dehydroxylation. This metabolic pathway converts CA to DCA or converts CDCA to LCA in eight steps. Abb. BaiB, bile acid-coenzyme A ligase; BaiA2, 3α-hydroxysteroid dehydrogenase; BaiCD, 7α-hydroxy-3-oxo-Δ4-cholenoic acid oxidoreductase; BaiE, bile acid 7α-dehydratase; BaiF, bile acid coenzyme A transferase/hydrolase; BaiH, 7β -hydroxy-3-oxo-Δ4-cholenoic acid oxidoreductase; CA, cholate; DCA, deoxycholate.
While the enzymes discussed above can sufficiently execute 7α-dehydroxylation, other enzymes are also capable of performing steps in this pathway, indicating some redundancy. Of particular interest is BaiA. While BaiA2 was the enzyme used to reconstruct the 7α-dehydroxylation pathway in vitro, another 3α-hydroxysteroid dehydrogenase called BaiA1 is also present in some of the bacteria that have demonstrated 7α-dehydroxylation capability [61]. BaiA1 is a close homolog of BaiA2 with 92% sequence identity that can also perform the oxidative step in the pathway [61]. BaiA1 has not been shown to catalyze the transformation from 3-oxo-DCA to DCA, but since Clostridium hylemonae TN 271 carries baiA1, but lacks baiA2 and has been shown to produce DCA, BaiA1 is likely able to perform both steps in the pathway, as C. hylemonae would be unable to produce DCA if baiA2 was necessary for 7α-dehydroxylation [67]. Clostridium hiranonis TO 931 carries baiA2, but lacks baiA1, while C. scindens ATCC 35704 and C. scindens VPI 12708 carry both [21]. C. scindens VPI 12708 also has a second copy of baiA1, referred to as baiA3 [68]. Another enzyme that is capable of performing steps in the 7α-dehydroxylation pathway is the flavoprotein BaiN, which is capable of converting 3-oxo-4,5-6,7-didehydro-DCA to 3-oxo-4,5-dehydro-DCA and then to 3-oxo-DCA, which are steps that can also be performed by BaiH and BaiCD, respectively [59,69].
While all organisms known to carry the bai operon have 7α-dehydroxylation activity, the regulation of the bai operon has yet to be fully elucidated [45,67,70,71]. C. scindens and C. hylemonae have increased expression of genes in the bai operon in defined media supplemented with CA, and C. hiranonis in rich media supplemented with CA [70,71]. While C. scindens also has increased expression of selected bai operon genes when grown in rich media, C. hylemonae does not, indicating differences in regulation of the bai operon between commensal Clostridium [57].
While most of the enzymes involved in 7α-dehydroxylation are not extensively characterized, BaiA and BaiE have both undergone structural and functional characterization [61,72]. The short chain dehydrogenase/reductase BaiA2 as well as the homolog BaiA1 shows exclusive preference for the cofactor NAD(H) rather than NADP(H), likely due to steric hindrance involving Glu42 in the cofactor binding site [61]. The dehydratase BaiE shows a preference for 3-oxo-Δ4-CDC-CoA over 3-oxo-Δ4-CDCA, with the Kcat/KM being an order of magnitude higher for the former than the latter, indicating that the 7α-dehydration step is more efficient when the intermediate is ligated to CoA [72].
3.3. Hydroxysteroid Dehydrogenases
Bacterial hydroxysteroid dehydrogenases (HSDHs) epimerize bile acid hydroxy groups on the 3-, 7-, or 12- carbons of bile acids in a two-step process requiring an α- and a β-HSDH that generates a stable oxo intermediate [21]. Commensal Clostridium can encode multiple HSDHs. Commensal Clostridium that encode the bai operon carry both a 7α-HSDH (baiA) as well as a 7β-HSDH [21]. Organisms with a 7α- and a 7β-HSDH can produce UDCA, which is the 7β-epimer of CDCA [21]. As UDCA is more hydrophilic and thus less toxic to gut bacteria than CDCA, the epimerization of CDCA using a 7β-HSDH could serve as a survival advantage for bacteria capable of accomplishing this transformation [21,73]. In addition, Ruminococcus gnavus carries a 3α-HSDH and Clostridium innocuum carries a 3β-HSDH [21,44,74]. The 3α/β epimerization of DCA and LCA creates iDCA and iLCA, respectively, which are the second most abundant secondary bile acids after DCA and LCA [22]. While no bacteria in the genus Clostridium have made iDCA, R. gnavus uses a 3α-HSDH to create the intermediate of 3-oxoDCA and then a 3β-HSDH to complete the transformation from DCA to iDCA [74]. iDCA exhibits reduced toxicity in vitro to some gut commensals including multiple species of Bacteroides and Clostridium sporogenes but has the ability to inhibit multiple strains of C. difficile at very low concentrations [24,74]. This indicates that the conversion from DCA to iDCA can serve to reduce toxicity for some commensals, as well as assisting the gut microbiota with colonization resistance against pathogens such as C. difficile. These same 3α- and 3β-HSDHs convert LCA to iLCA, which inhibits the growth of multiple strains of C. difficile in vitro at a lower concentration than LCA [24,74]. The toxicity of iLCA when compared to LCA on various commensals has yet to be determined, but it is possible that the epimerization of LCA to iLCA serves to reduce toxicity for some members of the gut microbiota, as well as assisting with colonization resistance against enteric pathogens such as C. difficile.
4. Bile Acids, Other Intestinal Pathogens, and the Host
While bile acids modified by commensal Clostridium affect the life cycle of C. difficile, they also have an inhibitory effect on other intestinal pathogens as well as a strong effect on the host. Bile acids can induce the transcription of genes responsible for DNA repair and recombination in E. coli, Salmonella enterica serovar Typhimurium, Bacillus cereus, and L. monocytogenes [75,76]. Genes responsible for maintaining the integrity of the cellular envelope are also upregulated in B. cereus and L. monocytogenes, indicating that bile acids damage the bacterial membrane and cellular DNA [76]. In particular, multiple strains of Shigella show a significant increase in biofilm formation and 143 genes have differential transcription when exposed to bile salts, which indicates a strong stress response [77]. Enteric pathogens have multiple bile resistance mechanisms including efflux pumps and DNA repair mechanisms, but bile acids are still important in colonization resistance against these intestinal pathogens [76].
Bile acids are important signaling molecules within the host as well. They interact primarily with the G-Protein-Coupled Bile Acid Receptor-1 (GPBAR-1, aka TGR5) and Farnesoid-X-Receptor alpha (FXRα) which belong to the nuclear receptor superfamily [17,18,21]. Secondary bile acids produced by commensal Clostridium are potent agonists for TGR5, specifically DCA and LCA [17,78]. TGR5 has been implicated in the regulation of multiple metabolic functions including glucose metabolism and the conversion of fat into energy, making it a potential target for treating obesity [17,78]. The most potent agonist for FXRα is CDCA, but DCA and LCA are also agonists for this receptor [17]. FXRα controls the enterohepatic circulation of bile acids and acts as an anti-inflammatory mediator in the liver and intestine, which could allow it to potentially help prevent tumor development [17]. However, high levels of the secondary bile acids DCA and LCA have been shown to correlate with tumors in the liver and intestine, specifically colon cancer [17,21]. High levels of DCA are also correlated with cholesterol gallstone disease in some patients [21]. The levels of bile acids can also affect FXR receptor expression, as giving mice exogenous UCDA increases the expression of TGR5 and FXR, causing alterations to the bile acid metabolome [43].
Bile acids are important not just for their effect on the gut microbiota and their contribution to colonization resistance against C. difficile and other intestinal pathogens, they are also important determinants of several other aspects of human health. Further studies examining the rational manipulation of bile acid pools and the effect of this alteration on colonization resistance against C. difficile and other intestinal pathogens are necessary and understanding the production of secondary bile acids by commensal Clostridium and other microbes is important for advancing our knowledge of human health and disease.
5. Production of Inhibitory Metabolites
While bile acids play a significant role in modulating the composition of the gut microbiota, there are other bacterial metabolites that can affect the gut microbiota and colonization resistance against C. difficile such as short-chain fatty acids (SCFAs) [38]. SCFAs are metabolized from fiber by commensal bacteria and the concentration of SCFAs are low in patient stool after taking broad spectrum antibiotics, in CDI patients, and in CDI-susceptible mice [38,79]. Increased levels of SCFAs are correlated with decreased tissue damage and immunomodulatory effects, making rational manipulation of SCFA production a potential strategy for targeted therapeutics against CDI. Increased levels of the SCFAs propionate, succinate, and butyrate were observed after FMT for recurrent CDI [38]. In addition, valerate inhibits C. difficile in a chemostat model, and butyrate can protect against C. difficile-induced colitis in the murine gut via reducing intestinal permeability and microbial translocation in an HIF-1 dependent fashion [42,80,81,82]. Members of the Clostridium cluster XIVa and IV are a significant source of butyrate production in the gut and are significantly depleted in the feces of patients with CDI or with nosocomial diarrhea (C. difficile negative) when compared to healthy control samples [80,81,82].
Despite the ability of multiple strains of C. difficile to generate butyrate, the presence of butyrate in the gut is associated with decreased fitness for C. difficile [83]. Specifically, when mice are fed microbiota-accessible carbohydrates, the SCFAs propionate, acetate, and butyrate increase, and the C. difficile burden decreases [83]. In addition, all three SCFAs negatively affect the growth of C. difficile, although all three SCFAs cause toxin expression to increase in vitro [83]. However, the overall level of toxin decreases due to the lower C. difficile burden in mice that are fed diets rich in microbiota-accessible carbohydrates [83].
Butyrate can be produced by bacteria through multiple pathways. The most common pathway in Clostridium is the synthesis of butyryl-CoA from acetyl-CoA and the subsequent liberation of butyrate from the CoA molecule [84]. There are multiple arrangements of the butyrate synthesis genes in Clostridium, with two arrangements being present in Cluster XIVa and a third distinct arrangement being present in butyrate producing Clostridium in Cluster I and Cluster XVI [84]. After butyryl-CoA is produced, the CoA moiety can be removed by butyryl-CoA/acetate CoA transferase (But) or the butyryl-CoA can be phosphorylated by phosphate butyryltransferase (Ptb), then transformed to butyrate by butyrate kinase (Buk), which generates ATP [85]. Most butyrate-producing Clostridium, including C. difficile contain Buk, some contain But instead, and a small number of strains encode both proteins [85].
However, lysine, glutarate, 4-aminobutyrate, and succinate can also serve as substrates for the production of butyrate. These three pathways are separate from the acetyl-CoA pathway, but all four pathways merge at the energy generating step where crotonyl-CoA is transformed into butyryl-CoA by the Bcd complex [85]. Multiple strains of C. difficile can generate butyrate using acetyl-CoA, 4-aminobutyrate, or succinate as a substrate. Clostridium sticklandii can use acetyl-CoA or lysine as a substrate [85,86]. The generation of butyrate from succinate by C. difficile is of particular interest as the ability to ferment succinate gives C. difficile a competitive advantage [86].
Antimicrobial compounds produced by members of the gut microbiota also affect colonization resistance against C. difficile. C. scindens ATCC 35704 produces 1-acetyl-β-carboline, a tryptophan-derived antibacterial compound that inhibits multiple Gram-positive pathogens found in the gut, including C. difficile, Staphylococcus aureus, and Clostridium sordellii [87]. While the specific mechanism of action is not known, cell division of C. difficile was inhibited and the additional presence of DCA or LCA enhanced the inhibitory effect of 1-acetyl-β-carboline in vitro [87].
6. Competition for Nutrients
Competition for nutrients also plays an important role in colonization resistance against C. difficile and other pathogens. Colonization of a susceptible murine host by a nontoxigenic strain of C. difficile protects against colonization by toxigenic C. difficile, indicating that colonization by bacteria with similar nutritional requirements can protect the host [88,89]. Strains of C. difficile belonging to the epidemic ribotypes (RT) 027 and 078 have gained the ability to metabolize low concentrations of trehalose, a common food additive [90]. In the RT 027 strain, a point mutation occurred that increased sensitivity to trehalose, while the RT 078 strain acquired additional genes that metabolize trehalose [90]. While the exact contribution to competitive fitness is unknown, the ability to metabolize trehalose increased virulence in a mouse model of C. difficile, indicating that increased ability to compete for trehalose in the gut may provide some form of competitive advantage [90]. C. difficile also uses sugar alcohols such as mannitol, N-acetylated amino acids, and carbohydrates during early infection in the murine gut, but the effect of each of those nutrients on competitive fitness is unknown [91].
Proline, hydroxyproline, and glycine are the most efficient electron acceptors for Stickland fermentation, while leucine, isoleucine, and alanine are the most efficient electron donors [27]. C. difficile is auxotrophic for isoleucine, leucine, and proline, and proline concentration affects the in vitro expression of genes in the prd operon which is responsible for proline reduction in Stickland fermentation [27,92]. Availability of these amino acids (alanine, glycine, leucine, isoleucine, and proline) in the gut correlates with increased susceptibility to CDI in a mouse model [93]. Proline in particular is important for C. difficile colonization as a prdB mutant is unable to use proline as an energy source. When a C. difficile prdB mutant was tested in a mouse model of CDI, the mice challenged with the prdB mutant had reduced colonization and a lower concentration of TcdB in their stool when compared to mice challenged with wild type C difficile, indicating that the ability to ferment proline is important for colonization and virulence [93]. In addition, when wild type C. difficile capable of fermenting proline and a prdB mutant were grown in the presence or absence of a commensal clostridia panel, the wild type C. difficile had a fitness advantage when the commensals were present, indicating that the presence of commensal clostridia increases reliance on proline fermentation [94]. However, when C. difficile competed with Paeniclostridium spp. or Clostridium xylanolyticum, two members of the commensal clostridia panel able to ferment proline, the competitive advantage conferred by wild type C. difficile in comparison to the prdB mutant was lower than when it was only competing with bacteria unable to ferment proline [94]. This indicates that C. difficile competes with commensal clostridia for proline. C. difficile also has a competitive advantage over C. scindens, C. hylemonae, and C. hiranonis in a rich media, although the extent to which this is due to the ability of C. difficile to ferment proline is unknown [57].
Hydroxyproline (Hyp) is a derivative of proline which has been post translationally modified by prolyl-4-hydroxylase [95]. It is important for stabilizing the triple helix structure in collagen, the most abundant mammalian protein [96]. It can be converted to proline in a two-step process that requires the hydroxyproline dehydratase HypD as well as the pyrroline-5-carboxylate reductase ProC, both of which are present in C. difficile [97,98]. Homologs of HypD are widespread in the gut microbiome, which suggests that the ability of bacteria to reduce hydroxyproline is useful in the gut [97]. However, of the bacteria encoding hypD, only a subset had an adjacent proC gene, indicating that the ability to reduce hydroxyproline to proline is not ubiquitous [97]. While Stickland fermentation of proline is important for C. difficile metabolism, it is not yet known how the reduction of hydroxyproline affects competitive fitness. However, the widespread presence of HypD and the competitive fitness advantage gained by proline fermentation in the presence of commensal clostridia indicates that it may play a significant role in the colonization of C. difficile in the gut [94,97].
7. Conclusions
There are several mechanisms of how the gut microbiota provides colonization resistance against C. difficile presented in this review, including the production of inhibitory metabolites, such as secondary bile acids, SCFAs, and antimicrobials, as well as competition for nutrients, especially proline and other amino acids necessary for Stickland fermentation. Understanding the mechanistic effects that these metabolites have on C. difficile and other gut pathogens is important for the development of new therapeutics against CDI, which are urgently needed.
Funding
A.D.R. was funded by the NCSU Molecular Biology Training Program T32 GM008776 through National Institutes of Health (NIH). C.M.T. was funded by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM119438.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
CMT is a consultant for Vedanta Biosciences and Summit Therapeutics.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hall I.C., O’Toole E. Intestinal flora in new-born infants: With a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Dis. Child. 1935;49:390–402. doi: 10.1001/archpedi.1935.01970020105010. [DOI] [Google Scholar]
- 2.Lessa F.C., Mu Y., Bamberg W.M., Beldavs Z.G., Dumyati G.K., Dunn J.R., Farley M.M., Holzbauer S.M., Meek J.I., Phipps E.C., et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill S.S., O’Leary E., Janelle S.J., Thompson D.L., Dumyati G., Nadle J., Wilson L.E., Kainer M.A., Lynfield R., Greissman S., et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018;379:1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fekety R., McFarland L.V., Surawicz C.M., Greenberg R.N., Elmer G.W., Mulligan M.E. Recurrent Clostridium difficile diarrhea: Characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin. Infect. Dis. 1997;24:324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 5.Cornely O.A., Miller M.A., Louie T.J., Crook D.W., Gorbach S.L. Treatment of first recurrence of Clostridium difficile infection: Fidaxomicin versus vancomycin. Clin. Infect. Dis. 2012;55(Suppl. 2):S154–S161. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens R.C., Jr., Donskey C.J., Gaynes R.P., Loo V.G., Muto C.A. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin. Infect. Dis. 2008;46(Suppl. 1):S19–S31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 7.Theriot C.M., Koenigsknecht M.J., Carlson P.E., Jr., Hatton G.E., Nelson A.M., Li B., Huffnagle G.B., Li J.Z., Young V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buffie C.G., Jarchum I., Equinda M., Lipuma L., Gobourne A., Viale A., Ubeda C., Xavier J., Pamer E.G. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect. Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theriot C.M., Young V.B. Interactions Between the Gastrointestinal Microbiome and Clostridium difficile. Annu. Rev. Microbiol. 2015;69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffie C.G., Bucci V., Stein R.R., McKenney P.T., Ling L., Gobourne A., No D., Liu H., Kinnebrew M., Viale A., et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livanos A.E., Snider E.J., Whittier S., Chong D.H., Wang T.C., Abrams J.A., Freedberg D.E. Rapid gastrointestinal loss of Clostridial Clusters IV and XIVa in the ICU associates with an expansion of gut pathogens. PLoS ONE. 2018;13:e0200322. doi: 10.1371/journal.pone.0200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solbach P., Chhatwal P., Woltemate S., Tacconelli E., Buhl M., Gerhard M., Thoeringer C.K., Vehreschild M., Jazmati N., Rupp J., et al. BaiCD gene cluster abundance is negatively correlated with Clostridium difficile infection. PLoS ONE. 2018;13:e0196977. doi: 10.1371/journal.pone.0196977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buffie C.G., Pamer E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducarmon Q.R., Zwittink R.D., Hornung B.V.H., van Schaik W., Young V.B., Kuijper E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019;83:e00007-19. doi: 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begley M., Gahan C.G., Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Molinero N., Ruiz L., Sánchez B., Margolles A., Delgado S. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinot E., Sedes L., Baptissart M., Lobaccaro J.M., Caira F., Beaudoin C., Volle D.H. Bile acids and their receptors. Mol. Asp. Med. 2017;56:2–9. doi: 10.1016/j.mam.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Wahlstrom A., Sayin S.I., Marschall H.U., Backhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann A.F. The Continuing Importance of Bile Acids in Liver and Intestinal Disease. Arch. Intern. Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 20.Falany C.N., Johnson M.R., Barnes S., Diasio R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 1994;269:19375–19379. doi: 10.1016/S0021-9258(17)32178-6. [DOI] [PubMed] [Google Scholar]
- 21.Ridlon J.M., Harris S.C., Bhowmik S., Kang D.J., Hylemon P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton J.P., Xie G., Raufman J.P., Hogan S., Griffin T.L., Packard C.A., Chatfield D.A., Hagey L.R., Steinbach J.H., Hofmann A.F. Human cecal bile acids: Concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G256–G263. doi: 10.1152/ajpgi.00027.2007. [DOI] [PubMed] [Google Scholar]
- 23.McNally L., Brown S.P. Building the microbiome in health and disease: Niche construction and social conflict in bacteria. Philos. Trans. R. Soc. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thanissery R., Winston J.A., Theriot C.M. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe. 2017;45:86–100. doi: 10.1016/j.anaerobe.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorg J.A., Sonenshein A.L. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 2008;190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sievers S., Metzendorf N.G., Dittmann S., Troitzsch D., Gast V., Troger S.M., Wolff C., Zuhlke D., Hirschfeld C., Schluter R., et al. Differential View on the Bile Acid Stress Response of Clostridioides difficile. Front. Microbiol. 2019;10:258. doi: 10.3389/fmicb.2019.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouillaut L., Self W.T., Sonenshein A.L. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J. Bacteriol. 2013;195:844–854. doi: 10.1128/JB.01492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nisman B., Raynaud M., Cohen G.N. Extension of the Stickland reaction to several bacterial species. Arch. Biochem. 1948;16:473. [PubMed] [Google Scholar]
- 29.Neumann-Schaal M., Jahn D., Schmidt-Hohagen K. Metabolism the Difficile Way: The Key to the Success of the Pathogen Clostridioides difficile. Front. Microbiol. 2019;10:219. doi: 10.3389/fmicb.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois T., Tremblay Y.D.N., Hamiot A., Martin-Verstraete I., Deschamps J., Monot M., Briandet R., Dupuy B. A microbiota-generated bile salt induces biofilm formation in Clostridium difficile. NPJ Biofilms Microbiomes. 2019;5:14. doi: 10.1038/s41522-019-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tam J., Icho S., Utama E., Orrell K.E., Gomez-Biagi R.F., Theriot C.M., Kroh H.K., Rutherford S.A., Lacy D.B., Melnyk R.A. Intestinal bile acids directly modulate the structure and function of C. difficile TcdB toxin. Proc. Natl. Acad. Sci. USA. 2020;117:6792–6800. doi: 10.1073/pnas.1916965117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrieze A., Out C., Fuentes S., Jonker L., Reuling I., Kootte R.S., van Nood E., Holleman F., Knaapen M., Romijn J.A. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014;60:824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Reeves A.E., Theriot C.M., Bergin I.L., Huffnagle G.B., Schloss P.D., Young V.B. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes. 2011;2:145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antunes L.C.M., Han J., Ferreira R.B., Lolić P., Borchers C.H., Finlay B.B. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob. Agents Chemother. 2011;55:1494–1503. doi: 10.1128/AAC.01664-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weingarden A.R., Chen C., Bobr A., Yao D., Lu Y., Nelson V.M., Sadowsky M.J., Khoruts A. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G310–G319. doi: 10.1152/ajpgi.00282.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giel J.L., Sorg J.A., Sonenshein A.L., Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS ONE. 2010;5:e8740. doi: 10.1371/journal.pone.0008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenigsknecht M.J., Theriot C.M., Bergin I.L., Schumacher C.A., Schloss P.D., Young V.B. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect. Immun. 2015;83:934–941. doi: 10.1128/IAI.02768-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seekatz A.M., Theriot C.M., Rao K., Chang Y.M., Freeman A.E., Kao J.Y., Young V.B. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe. 2018;53:64–73. doi: 10.1016/j.anaerobe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown J.R.-M., Flemer B., Joyce S.A., Zulquernain A., Sheehan D., Shanahan F., O’Toole P.W. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018;18:1–15. doi: 10.1186/s12876-018-0860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farowski F., Solbach P., Tsakmaklis A., Brodesser S., Aguilar M.R.C., Cornely O.A., Dettmer K., Higgins P.G., Suerbaum S., Jazmati N. Potential biomarkers to predict outcome of faecal microbiota transfer for recurrent Clostridioides difficile infection. Dig. Liver Dis. 2019;51:944–951. doi: 10.1016/j.dld.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Kitahara M., Takamine F., Imamura T., Benno Y. Assignment of Eubacterium sp. VPI 12708 and related strains with high bile acid 7alpha-dehydroxylating activity to Clostridium scindens and proposal of Clostridium hylemonae sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2000;50(Pt 3):971–978. doi: 10.1099/00207713-50-3-971. [DOI] [PubMed] [Google Scholar]
- 42.Amrane S., Bachar D., Lagier J.C., Raoult D. Clostridium scindens Is Present in the Gut Microbiota during Clostridium difficile Infection: A Metagenomic and Culturomic Analysis. J. Clin. Microbiol. 2018;56 doi: 10.1128/JCM.01663-17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Winston J.A., Rivera A.J., Cai J., Thanissery R., Montgomery S.A., Patterson A.D., Theriot C.M. Ursodeoxycholic Acid (UDCA) Mitigates the Host Inflammatory Response during Clostridioides difficile Infection by Altering Gut Bile Acids. Infect. Immun. 2020;88 doi: 10.1128/IAI.00045-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Narushima S., Itoha K., Miyamoto Y., Park S.H., Nagata K., Kuruma K., Uchida K. Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids. 2006;41:835–843. doi: 10.1007/s11745-006-5038-1. [DOI] [PubMed] [Google Scholar]
- 46.Begley M., Hill C., Gahan C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinn R.A., Melnik A.V., Vrbanac A., Fu T., Patras K.A., Christy M.P., Bodai Z., Belda-Ferre P., Tripathi A., Chung L.K. Global chemical effects of the microbiome include new bile-acid conjugations. Nature. 2020;579:123–129. doi: 10.1038/s41586-020-2047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huijghebaert S.M., Mertens J., Eyssen H.J. Isolation of a bile salt sulfatase-producing Clostridium strain from rat intestinal microflora. Appl. Environ. Microbiol. 1982;43:185–192. doi: 10.1128/AEM.43.1.185-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bushman F.D., Conrad M., Ren Y., Zhao C., Gu C., Petucci C., Kim M.-S., Abbas A., Downes K.J., Devas N. Multi-omic analysis of the interaction between Clostridioides difficile infection and pediatric inflammatory bowel disease. Cell Host Microbe. 2020;28:422–433.e7. doi: 10.1016/j.chom.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka H., Hashiba H., Kok J., Mierau I. Bile salt hydrolase of Bifidobacterium longum—biochemical and genetic characterization. Appl. Environ. Microbiol. 2000;66:2502–2512. doi: 10.1128/AEM.66.6.2502-2512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tannock G.W., Dashkevicz M.P., Feighner S.D. Lactobacilli and bile salt hydrolase in the murine intestinal tract. Appl. Environ. Microbiol. 1989;55:1848–1851. doi: 10.1128/AEM.55.7.1848-1851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dussurget O., Cabanes D., Dehoux P., Lecuit M., Consortium E.L.G., Buchrieser C., Glaser P., Cossart P. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 2002;45:1095–1106. doi: 10.1046/j.1365-2958.2002.03080.x. [DOI] [PubMed] [Google Scholar]
- 53.De Smet I., Van Hoorde L., Vande Woestyne M., Christiaens H., Verstraete W. Significance of bile salt hydrolytic activities of lactobacilli. J. Appl. Bacteriol. 1995;79:292–301. doi: 10.1111/j.1365-2672.1995.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 54.Foley M.H., O’Flaherty S., Allen G.B., Stewart A., Barrangou R. Lactobacillus Bile Salt Hydrolase Substrate Specificity Governs Bacterial Fitness and Host Colonization. Proc. Natl. Acad. Sci. USA. 2021;118:e2017709118. doi: 10.1073/pnas.2017709118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofmann A.F., Mysels K.J. Bile acid solubility and precipitation in vitro and in vivo: The role of conjugation, pH, and Ca2+ ions. J. Lipid Res. 1992;33:617–626. doi: 10.1016/S0022-2275(20)41426-9. [DOI] [PubMed] [Google Scholar]
- 56.Hofmann A.F., Roda A. Physicochemical properties of bile acids and their relationship to biological properties: An overview of the problem. J. Lipid Res. 1984;25:1477–1489. doi: 10.1016/S0022-2275(20)34421-7. [DOI] [PubMed] [Google Scholar]
- 57.Reed A.D., Nethery M.A., Stewart A., Barrangou R., Theriot C.M. Strain-dependent inhibition of Clostridioides difficile by commensal Clostridia encoding the bile acid inducible (bai) operon. J. Bacteriol. 2020 doi: 10.1128/JB.00039-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullish B.H., McDonald J.A., Pechlivanis A., Allegretti J.R., Kao D., Barker G.F., Kapila D., Petrof E.O., Joyce S.A., Gahan C.G. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut. 2019;68:1791–1800. doi: 10.1136/gutjnl-2018-317842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Funabashi M., Grove T.L., Pascal V., Varma Y., McFadden M.E., Brown L.C., Guo C., Medema M.H., Almo S.C., Fischbach M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582:566–570. doi: 10.1038/s41586-020-2396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mallonee D.H., Hylemon P.B. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J. Bacteriol. 1996;178:7053–7058. doi: 10.1128/JB.178.24.7053-7058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhowmik S., Jones D.H., Chiu H.P., Park I.H., Chiu H.J., Axelrod H.L., Farr C.L., Tien H.J., Agarwalla S., Lesley S.A. Structural and functional characterization of BaiA, an enzyme involved in secondary bile acid synthesis in human gut microbe. Proteins. 2014;82:216–229. doi: 10.1002/prot.24353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mallonee D.H., Adams J.L., Hylemon P.B. The bile acid-inducible baiB gene from Eubacterium sp. strain VPI 12708 encodes a bile acid-coenzyme A ligase. J. Bacteriol. 1992;174:2065–2071. doi: 10.1128/JB.174.7.2065-2071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang D.J., Ridlon J.M., Moore D.R., 2nd, Barnes S., Hylemon P.B. Clostridium scindens baiCD and baiH genes encode stereo-specific 7alpha/7beta-hydroxy-3-oxo-delta4-cholenoic acid oxidoreductases. Biochim. Biophys. Acta. 2008;1781:16–25. doi: 10.1016/j.bbalip.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dawson J.A., Mallonee D.H., Bjorkhem I., Hylemon P.B. Expression and characterization of a C24 bile acid 7 alpha-dehydratase from Eubacterium sp. strain VPI 12708 in Escherichia coli. J. Lipid Res. 1996;37:1258–1267. doi: 10.1016/S0022-2275(20)39155-0. [DOI] [PubMed] [Google Scholar]
- 65.Ye H.Q., Mallonee D.H., Wells J.E., Bjorkhem I., Hylemon P.B. The bile acid-inducible baiF gene from Eubacterium sp. strain VPI 12708 encodes a bile acid-coenzyme A hydrolase. J. Lipid Res. 1999;40:17–23. doi: 10.1016/S0022-2275(20)33335-6. [DOI] [PubMed] [Google Scholar]
- 66.Ridlon J.M., Hylemon P.B. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7 -dehydroxylating intestinal bacterium. J. Lipid Res. 2012;53:66–76. doi: 10.1194/jlr.M020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ridlon J.M., Kang D.J., Hylemon P.B. Isolation and characterization of a bile acid inducible 7alpha-dehydroxylating operon in Clostridium hylemonae TN271. Anaerobe. 2010;16:137–146. doi: 10.1016/j.anaerobe.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gopal-Srivastava R., Mallonee D.H., White W.B., Hylemon P.B. Multiple copies of a bile acid-inducible gene in Eubacterium sp. strain VPI 12708. J. Bacteriol. 1990;172:4420–4426. doi: 10.1128/JB.172.8.4420-4426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris S.C., Devendran S., Alves J.M.P., Mythen S.M., Hylemon P.B., Ridlon J.M. Identification of a gene encoding a flavoprotein involved in bile acid metabolism by the human gut bacterium Clostridium scindens ATCC 35704. Biochim. Biophys. Acta. 2018;1863:276–283. doi: 10.1016/j.bbalip.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Ridlon J.M., Devendran S., Alves J.M., Doden H., Wolf P.G., Pereira G.V., Ly L., Volland A., Takei H., Nittono H., et al. The ‘in vivo lifestyle’ of bile acid 7alpha-dehydroxylating bacteria: Comparative genomics, metatranscriptomic, and bile acid metabolomics analysis of a defined microbial community in gnotobiotic mice. Gut Microbes. 2019:1–24. doi: 10.1080/19490976.2019.1618173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devendran S., Shrestha R., Alves J.M.P., Wolf P.G., Ly L., Hernandez A.G., Mendez-Garcia C., Inboden A., Wiley J., Paul O., et al. Clostridium scindens ATCC 35704: Integration of Nutritional Requirements, the Complete Genome Sequence, and Global Transcriptional Responses to Bile Acids. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.00052-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhowmik S., Chiu H.P., Jones D.H., Chiu H.J., Miller M.D., Xu Q., Farr C.L., Ridlon J.M., Wells J.E., Elsliger M.A., et al. Structure and functional characterization of a bile acid 7alpha dehydratase BaiE in secondary bile acid synthesis. Proteins. 2016;84:316–331. doi: 10.1002/prot.24971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heuman D.M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 1989;30:719–730. doi: 10.1016/S0022-2275(20)38331-0. [DOI] [PubMed] [Google Scholar]
- 74.Devlin A.S., Fischbach M.A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 2015;11:685–690. doi: 10.1038/nchembio.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merritt M.E., Donaldson J.R. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J. Med. Microbiol. 2009;58:1533–1541. doi: 10.1099/jmm.0.014092-0. [DOI] [PubMed] [Google Scholar]
- 76.Urdaneta V., Casadesus J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017;4:163. doi: 10.3389/fmed.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nickerson K.P., Chanin R.B., Sistrunk J.R., Rasko D.A., Fink P.J., Barry E.M., Nataro J.P., Faherty C.S. Analysis of Shigella flexneri Resistance, Biofilm Formation, and Transcriptional Profile in Response to Bile Salts. Infect. Immun. 2017;85 doi: 10.1128/IAI.01067-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fiorucci S., Biagioli M., Zampella A., Distrutti E. Bile acids activated receptors regulate innate immunity. Front. Immunol. 2018;9:1853. doi: 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fachi J.L., Felipe J.S., Pral L.P., da Silva B.K., Correa R.O., de Andrade M.C.P., da Fonseca D.M., Basso P.J., Camara N.O.S., de Sales E.S.E.L., et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019;27:750–761.e7. doi: 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 80.Riviere A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van den Abbeele P., Belzer C., Goossens M., Kleerebezem M., De Vos W.M., Thas O., De Weirdt R., Kerckhof F.M., Van de Wiele T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–961. doi: 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antharam V.C., Li E.C., Ishmael A., Sharma A., Mai V., Rand K.H., Wang G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013;51:2884–2892. doi: 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hryckowian A.J., Van Treuren W., Smits S.A., Davis N.M., Gardner J.O., Bouley D.M., Sonnenburg J.L. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat. Microbiol. 2018;3:662–669. doi: 10.1038/s41564-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Louis P., McCrae S.I., Charrier C., Flint H.J. Organization of butyrate synthetic genes in human colonic bacteria: Phylogenetic conservation and horizontal gene transfer. FEMS Microbiol. Lett. 2007;269:240–247. doi: 10.1111/j.1574-6968.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 85.Vital M., Howe A.C., Tiedje J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio. 2014;5:e00889-14. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferreyra J.A., Wu K.J., Hryckowian A.J., Bouley D.M., Weimer B.C., Sonnenburg J.L. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe. 2014;16:770–777. doi: 10.1016/j.chom.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang J.D., Myers C.J., Harris S.C., Kakiyama G., Lee I.K., Yun B.S., Matsuzaki K., Furukawa M., Min H.K., Bajaj J.S., et al. Bile Acid 7alpha-Dehydroxylating Gut Bacteria Secrete Antibiotics that Inhibit Clostridium difficile: Role of Secondary Bile Acids. Cell Chem. Biol. 2019;26:27–34.e4. doi: 10.1016/j.chembiol.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson K.H., Sheagren J.N. Antagonism of toxigenic Clostridium difficile by nontoxigenic C. difficile. J. Infect. Dis. 1983;147:733–736. doi: 10.1093/infdis/147.4.733. [DOI] [PubMed] [Google Scholar]
- 89.Gerding D.N., Meyer T., Lee C., Cohen S.H., Murthy U.K., Poirier A., Van Schooneveld T.C., Pardi D.S., Ramos A., Barron M.A., et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: A randomized clinical trial. JAMA. 2015;313:1719–1727. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 90.Collins J., Robinson C., Danhof H., Knetsch C.W., van Leeuwen H.C., Lawley T.D., Auchtung J.M., Britton R.A. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature. 2018 doi: 10.1038/nature25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fletcher J.R., Erwin S., Lanzas C., Theriot C.M. Shifts in the Gut Metabolome and Clostridium difficile Transcriptome throughout Colonization and Infection in a Mouse Model. mSphere. 2018;3 doi: 10.1128/mSphere.00089-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karasawa T., Ikoma S., Yamakawa K., Nakamura S. A defined growth medium for Clostridium difficile. Pt 2Microbiology. 1995;141:371–375. doi: 10.1099/13500872-141-2-371. [DOI] [PubMed] [Google Scholar]
- 93.Battaglioli E.J., Hale V.L., Chen J., Jeraldo P., Ruiz-Mojica C., Schmidt B.A., Rekdal V.M., Till L.M., Huq L., Smits S.A., et al. Clostridioides difficile uses amino acids associated with gut microbial dysbiosis in a subset of patients with diarrhea. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aam7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopez C.A., McNeely T.P., Nurmakova K., Beavers W.N., Skaar E.P. Clostridioides difficile proline fermentation in response to commensal clostridia. Anaerobe. 2020;63:102210. doi: 10.1016/j.anaerobe.2020.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gorres K.L., Raines R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Lullo G.A., Sweeney S.M., Körkkö J., Ala-Kokko L., San Antonio J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002;277:4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 97.Huang Y.Y., Martinez-Del Campo A., Balskus E.P. Anaerobic 4-hydroxyproline utilization: Discovery of a new glycyl radical enzyme in the human gut microbiome uncovers a widespread microbial metabolic activity. Gut Microbes. 2018;9:437–451. doi: 10.1080/19490976.2018.1435244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levin B.J., Huang Y.Y., Peck S.C., Wei Y., Martinez-Del Campo A., Marks J.A., Franzosa E.A., Huttenhower C., Balskus E.P. A prominent glycyl radical enzyme in human gut microbiomes metabolizes trans-4-hydroxy-l-proline. Science. 2017;355 doi: 10.1126/science.aai8386. [DOI] [PMC free article] [PubMed] [Google Scholar]