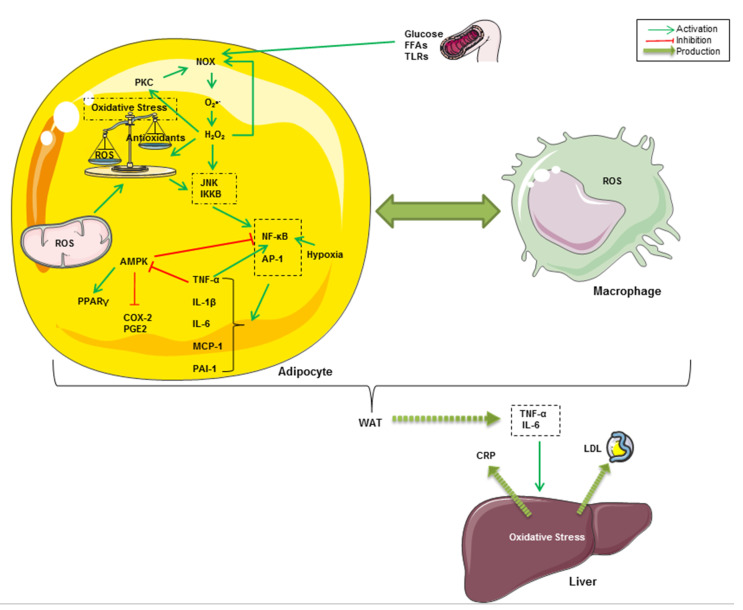
Figure 1.
Graphical summary of some inflammatory and oxidative pathways related to obesity. High glucose intake and FFA-dependent TLR4 activation lead to the activation of NOX, which can also be activated by PKC in adipocytes. NOX mediates intracellular ROS generation, mainly O2•−, both in preadipocytes and macrophages. Likewise, mitochondrial ROS exacerbate oxidative stress and inflammatory processes in obesity. JNK mediates ROS and oxidative stress-dependent activation of NF-κB and AP-1. These latters regulate gene expression of TNF-α, IL-6, MCP-1 and PAI-1. The NF‑κB pathway might be upregulated in hypoxic adipose tissue and in response to TNF-α. Furthermore, TNF-α inhibits AMPK pathways, resulting in increased COX-2 and PGE2 and decreased PPARγ. TNF-α and IL-6 secreted from WAT enhance CRP and low-density lipoprotein (LDL) release from the liver in response to hepatic oxidative stress. A crosstalk between adipocytes and resident macrophages reinforces oxidative stress through TNF-α–mediated ROS generation within WAT cells in an autocrine and paracrine manner.