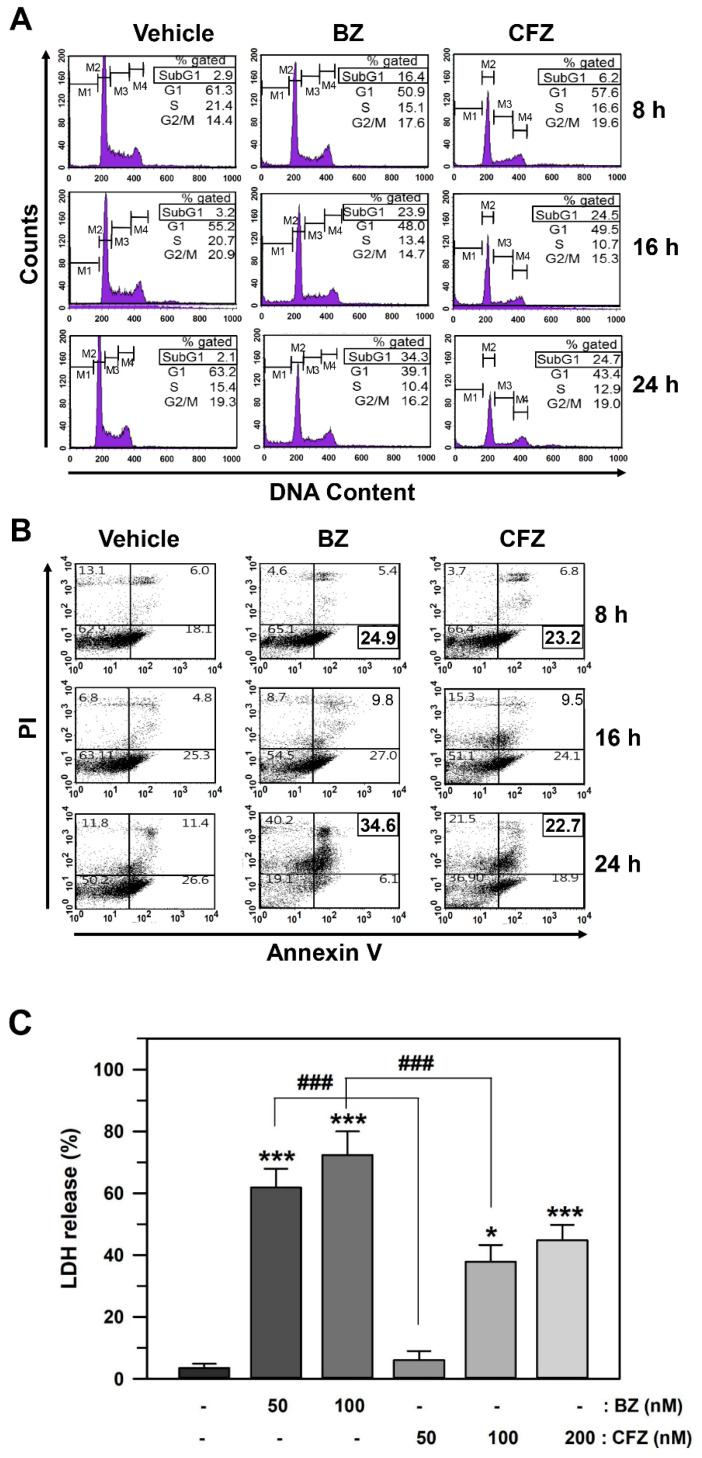
Figure 2.
Effect of BZ and CFZ on apoptosis in B16-F1 cells. (A,B) Cells were treated with vehicle or 50 nM BZ or 100 nM CFZ in the culture medium for the indicated times. Ethanol-fixed cells were stained with propidium idodide (PI) for DNA fragmentation detection (A) and intact cells were double-stained with PI and annexin V-FITC for apoptosis detection (B). The fluorescence was evaluated by flow cytometry and the percentage of cells was calculated (n = 9 for total replicates). The average values are shown in the upper corner of each area. (C) The cytotoxicity was determined by LDH release assay and the percent cytotoxicity is plotted as means ± standard deviations (n = 12 for total replicates). The statistically significant difference between groups was determined by one-way ANOVA followed by Dunnett’s T3 post hoc test. * p < 0.05 and *** p < 0.001 compared with the vehicle-treated control. ### p < 0.001 compared between BZ and CFZ treatment groups at equal concentration. (A–C) Cells were treated with various concentrations of BZ or CFZ for 24 h in 2% FBS/DMEM or 10% FBS/DMEM, or with 100 nM BZ or CFZ for the indicated times in 2% FBS/DMEM (F). Total cell extracts were analyzed by Western blotting with antibodies against the cleaved forms of caspases (Cas 3, 8, 9, and 12) and β-actin. The abbreviation (C) after the name of caspases stands for “cleaved”. The band densities were normalized against β-actin (n = 6 for total replicates) and the fold changes compared to that of vehicle-treated control (0 nM in A and B, −/− at 4 h in C) are written under each band.