Abstract
Background:
Cow’s milk intolerance can lead to chronic constipation in children. The present study seeks to determine the effect of cow’s milk-free diet (CMFD) on chronic constipation in children who are not responding to laxatives.
Methods:
Seventy children suffering from chronic constipation (described as the Rome III criteria) were enrolled in an open-label randomized clinical study. Each group included 35 children aged 4-14 years treated with laxatives for at least three months with no improvements. The intervention group received CMFD plus calcium supplements for four weeks and the control group did not have any restrictions in consuming cow’s milk and dairy products. Also, both groups received polyethylene glycol (PEG; 1 gr/kg/day) and high-fiber foods (at least 10 gr/day) for four weeks. Responsiveness was described as a reduction in symptoms and signs according to the Rome III criteria after four weeks.
Results:
After four weeks, 25 (71.4%) children in the CMFD group responded to the treatment compared to four (11.4%) children in the control group (P<0.001). Significant differences were found between the CMFD and control groups in terms of the seven Rome III criteria post-intervention; history of large stools (25% vs. 53.6%), large fecal mass in the rectum during examination (17.1% vs. 50%), history of painful defecation (18.2% vs. 55.6%), history of retentive posturing (10% vs. 46%), ≥1 episode/week of incontinence (25 % vs. 50%, P=0.001), ≤ 2 defecations/week (17.4% vs. 52.3%) and history of thick stool with toilet obstruction (22.2% vs. 52.3%)
Conclusion:
This study showed that children with functional constipation with no response to laxatives could benefit from a cow’s milk-free and dairy-free diet.
Key Words: Cow’s milk, Allergy, Constipation, Children
Chronic constipation is one of the most common pediatric conditions with a frequency of 3% to 16%. Despite a high prevalence, the cause of chronic constipation is still unknown (1, 2). The first therapeutic approaches to treating this malady are nutritional modification and a diet containing fiber or laxatives. It is necessary to first rule out the chance of the condition being secondary to another medical condition, such as hypothyroidism and congenital gastrointestinal disease, or having a reaction to any medications (3). Although the most common gastrointestinal symptom of cow’s milk intolerance in children is chronic diarrhea (4), there have been reports of a link between cow’s milk allergy (CMA) and chronic constipation (2, 5-7). It is supposed that cow’s milk intolerance can lead to severe perianal lesions and painful defecation and consequent constipation (8). A systematic review with a total of 505 patients resulted that a cow’s milk–free diet was effective in a percentage of children, varying from 28% to 78% (5).
Iacono et al. (8) suggested a relationship between the constipation and cow’s milk intake in about two-thirds of constipated children, but study of Simeone were against this finding (9). It is possible CMA is one of the reasons for not responding to laxative treatment in children with chronic constipation (4, 8, 10). So, the present study was conducted to evaluate the effect of a cow’s milk-free diet (CMFD) on children with chronic constipation not responding to standard treatments.
Methods
An open-label-randomized clinical trial performed on children suffering from chronic constipation referred to the pediatric gastroenterology clinic of Amirkola Children’s Hospital between June 2018 and June 2019. The children were evaluated based on the Rome Ш criteria for the diagnosis of pediatric chronic constipation. The inclusion criteria were: (i) Being aged 4-14 years; (ii) Taking high-fiber foods at least 10 gr/day; (iii) No response to PEG after a three-month treatment period; (iv) Having no red flag symptoms: delayed passage of meconium, vomiting, fever, diarrhea, severe abdominal distension, rectal bleeding (unless attributable to an anal fissure), constipation present from birth or early infancy, urinary incontinence or bladder disease, ribbon stools, delayed growth, weight loss or poor weight gain, extraintestinal symptoms (especially neurologic deficits, physical findings suggesting possible anorectal disorder, congenital syndromes or anomalies related to Hirschsprung disease (3). The exclusion criteria were: (i) Having anatomical reasons for constipation, such as hirschsprung disease and spinal disease; (ii) A history of anal surgery; (iii) A history of UTI and urinary incontinence; (iv) The use of medications causing constipation; (v) Constipation because of other disorders, for example psychomotor retardation, hypothyroidism, cystic fibrosis and celiac disease, etc. Informed consent was gained from all children’s parents. The study was pre-registered on the Iranian Registry of Clinical Trials website (IRCT ID: 20190306042946N1) and approved by the ethics committee of Babol University of Medical Sciences (MUBABOL.HRI.REC.1397.291).
According to study of Dehghani et al. (3), defecations per week ≥ 2 was 28% in children with CMFD and 10% for control group, with the confidence of 95% and test power of 84%, 35 children were estimated as the sample size in each group. At baseline, a physical examination was performed, and information including breastfeeding during infancy, atopic disease in the children and their family and family history of constipation were recorded. The initial biochemical evaluation examined celiac disease, hypothyroidism, blood urea nitrogen (BUN) creatinine (Cr), urine analysis and CBC with eosinophil count and serum IgE concentrations. Anorectal manometry was also performed. Seventy children who met the eligibility criteria were randomly assigned into two groups through a computer-generated randomization code. The intervention group or group A received a cow’s milk-free and dairy-free diet plus 30 mg/kg/day of calcium syrup (Calciram, Ramo Pharmin Company, IR Iran) for four consecutive weeks. Group B or the control group did not have any restrictions in consuming cow’s milk and dairy products. Polyethylene glycol (PEG; 1gr/kg/day) was administered to both groups for four weeks. The children in both groups were assigned to two age subgroups, including a subgroup less than age 7 years and a subgroup above this age.
During the study, the children’s parents were asked to record the frequency of their children’s defecation per week, incontinency per week, difficulty in passing stool, stools with large diameter, which can barricade the retentive posturing and toilet. They were advised to contact the therapist if their children experienced any signs and symptoms such as nausea, vomiting, diarrhea, abdominal pain and skin symptoms. At the end of the study, i.e. after four weeks, the patients were re-examined in the gastroenterology clinic and re-assessed for anal fissure and meeting the Rome III criteria. The primary outcome was response to the treatment regimen based on the Rome Ш criteria (i.e. not meeting the criteria anymore) after the intervention and the secondary outcome was recovery of anal fissure.
Collected data were conducted using the SPSS 22.0 software. Percentages and frequencies were used to define categorical variables and standard deviations and mean to express the normally-distributed continuous variables. Fisher’s Exact test or Pearson’s chi-square test (categorical variables) or the Student t-test or Mann-Whitney’s test (continuous variables) were used to examine and compare the Rome III criteria within or between the groups.
Results
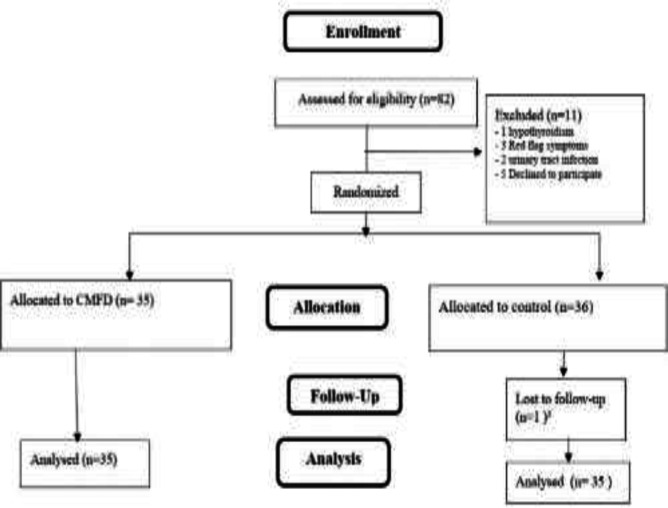
This study involved 70 children with functional constipation, aged 4 to 14 years. Figure 1 illustrates the number of persons participated in the study for assessment through follow-up.
Figure1.
Consort diagram
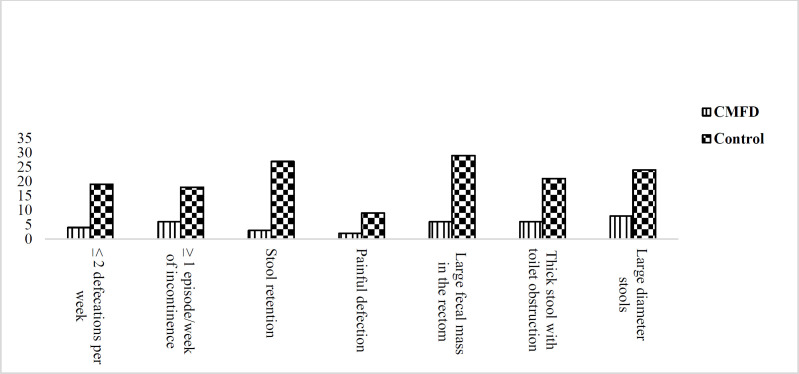
The two groups were matched for age, gender, weight and breastfeeding in infancy. There was no significant difference between the subjects in baseline characteristic (table 1). After a four-week trial, 25 (71.4%) of the patients responded to the CMFD in comparison to the four (11.4%) patients in the control group (P<0.001). No significant differences were found between the intervention and control groups in terms of any of the seven Rome III criteria before the trial, including history of stools with large diameter (53.6% vs. 46.4%, P=0.23), presence of a large fecal mass in the rectum during the examination (50% vs. 50%, P=0.99), history of hard or painful bowel movements (55.6% vs. 44.4%, P=0.58), history of retentive posturing (46% vs. 54%, P=0.29), ≥1 episode/week of incontinence (50 % vs. 50%, P=0.99), ≤ 2 defecations per week (52.3% vs. 47.7%, P=0.62) and history of thick stool with toilet obstruction (52.3% vs. 47.7%, P=0.62). The two groups were not significantly different in terms of abdominal pain (P=0.31), eosinophil count (P=0.23), pulmonary (P=0.59) and gastrointestinal allergies (P=0.99) and high IgE (P=0.70). Table 2 and figure 1 present the post-intervention comparison of the Rome III criteria within the CMFD group and between the two groups, respectively. Anal fissure improved in nine of the ten children in the CMFD group, but there was no recovery in this regard in the control group (P=0.01).
Table 1.
Clinical characteristics of children with chronic constipation before trial
| Variables | Children on cow milk free diet (N=35) | Children on cow milk diet (N=35) | P.value |
|---|---|---|---|
| Age [mean ±SD] (years) | 5±2 | 6 ±2 | 0.07 |
| Sex (Male%) | 19 (54 %) | 18 (51 %) | 0.81 |
| Age ≤ 7 years | 27(55.1 %) | 22(44.9 %) | 0.19 |
| Weight [mean ±SD] (kg) | 21.8±o.28 | 22.9±0.85 | 0.42 |
| Breast feeding at infancy (%) | 22 (47.8 %) | 24(52.2%) | 0.87 |
| Family history of constipation | 10(28 %) | 4(11%) | 0.07 |
| History of atopic disease | 2 (5 %) | 2 (5%) | 0.69 |
| Family history of atopic disease | 3 (8 %) | 0 | 0.07 |
| Anal fissure | 10(28 %) | 8(22 %) | 0.58 |
Table 2.
Comparison of Rome criteria between CMFD and control groups 4 weeks after trial
| Variables (N %) | Children on cow milk free diet before trial (N=35) | Children on cow milk diet after trial (N=35) | P.value |
|---|---|---|---|
| ≤ 2 defecations per week | 23 (52.3) | 4 (17.4) | <0.001 |
| ≥ 1 episode/week of incontinence after the acquisition of toileting skills | 19 (50) | 6 (25) | 0.001 |
| History of retentive posturing or excessive volitional stool retention |
23(46) | 3 (10) | <0.001 |
| History of painful defection or hard bowel movements | 10(55.6) | 2 (18.2) | 0.004 |
| Presence of a large fecal mass in the rectum | 33 (50) | 6 (17.1) | <0.001 |
| History of thick stool with toilet obstruction | 23(52.3) | 6 (22.2) | <0.001 |
| History of large diameter stools | 30 (53.6) | 8 (25) | <0.001 |
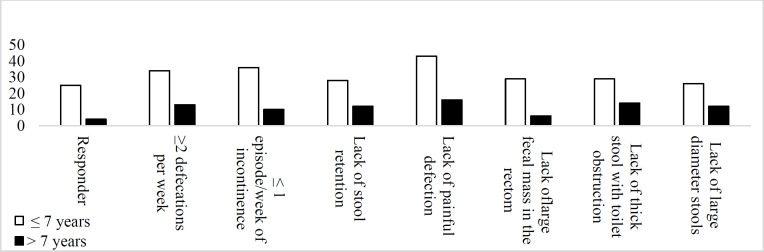
Table 2 and figure2,3 presents a comparison of the two age subgroups that there was significant difference between two groups in lack of large-diameter stools (P>0.001), defecations >2 per week (P>0.001), lack of a large fecal mass in the rectum during the examination (P=0.02), painless defecations (P>0.001), lack of incontinence per week (P=0.004) and lack of thick stool with toilet obstruction (P<0.001), after intervention.
Figure.2.
Comparison Rome III criteria between children in CMFD and control groups after trial
Figure 3.
Comparison Rome III criteria between two age group in CMFD and control groups after trial
Discussion
This study showed that a cow's milk protein-free diet improved all the seven Rome III criteria significantly. Cow’s milk proteins slow down bowel movement and inflammation and the increased eosinophilia as a result of this protein increases the internal anal sphincter resting pressure and thus leading to constipation and anal fissure (2, 11-15). Hypersensitivity to cow's milk protein is one of the causes of constipation in children; therefore it seems that the elimination of dairy products should improve constipation. Allergy to cow's milk proteins is more common in the age group of less than 7 years (16). Walker et al. also found that food allergies, especially CMA, decreases with age (17). In the present study, 70% of the children with functional constipation were in this age group, and of the 29 children who responded to the CMFD, 25 (86.2%) were less than seven years old, which indicates a higher prevalence of allergy to cow's milk proteins and thereby functional constipation in this age group.
Several studies found similar results. Responsiveness to the elimination of cow's milk from the diet for four weeks was 80% in a study by Dehghani et al.(3) and 77% in the study by El-Hodhod et al. (18). In another study, 28-78% of the constipated children responded to CMFD (6). Another study found that having a high-dairy and low-fiber diet leads to constipation in children (19). In the present study, however, fiber consumption was similar in both groups and the dairy restriction imposed in one of the groups improved their constipation, suggesting that a diet of cow’s milk and dairy products could be a reason for treatment-resistant constipation.
Iacono et al. and Daher found that children with constipation who improved after a CMFD had high serum IgE antibodies and positive skin prick test (1, 20). In a study by Irastorza et al. (2) constipation resolved within one to five days of the elimination of dairy and relapsed with the restart of dairy, suggests the role of late allergic reactions. The findings of another study showed a relationship between the constipation and consumption of cow’s milk in two-thirds of the children and revealed that hard stools or painful defecation reappeared after five to ten days of restarting the diet containing cow's milk (8). Andıran et al. (21) showed neonates and young children with anal fissure and chronic constipation compared to those with a normal bowel habit consume larger amounts of cow's milk. Miceli Sopo et al. (5) and Turunen et al. (22) argued that the avoidance of cow’s milk protein for two to four weeks may be a good treatment for children with constipation. In contrast, Simeone et al. (9) found no improvements in 11 children with constipation after four weeks of the CMFD. This disparity of findings could be due to the differences in the number and age of the studied samples as well as their age at the onset of constipation. Another study found no differences between the responder and non-responder groups of CMFD in terms of history of atopic allergies and biochemical test results (2). The evidence on allergy to cow’s milk protein in children with functional constipation is thus conflicting; a causal relationship between constipation and cow’s milk should be further investigated by a double-blind, crossover, placebo controlled trial. There are several other questions as well; for example, should children with constipation be tested for CMA? And how accurate are these tests? What are the reasons that could justify allergy testing in these children?
This study has some limitations. It was a clinical trial without blinding. Moreover, psychological factors indicated as the cause of chronic constipation could not be totally ignored in our study. Other limitations are the lack of challenge test and long-term follow-up of patients in terms of the possibility of recurrence of symptoms. It may have overestimated the frequency of CMA in constipated children because our gastroenterology center is a referral center and all patients who were treated unsuccessfully with laxatives were referred there.
In conclusion this study showed that the omission of dairy and cow’s milk from the diet is recommended for all children suffering from functional constipation unresponsive to laxative and fiber treatments.
Acknowledgments
The authors would like to thank the Research Deputy of Babol University of Medical Sciences, Non-Communicable Pediatric Diseases Research Center as well as the children and parents who participated in this research.
Funding:
This study was supported by a research grant and residency thesis of Dr Atena Mohammadi Bourkheili from the Non-Communicable Pediatric Diseases Research Center of Babol University of Medical Sciences (Grant Number: 9706940).
Conflicts of interest:
The authors declare that they have no conflict of interest.
References
- 1.Iacono G, Bonventre S, Scalici C, et al. Food intolerance and chronic constipation: manometry and histology study. Eur J Gastroenterol Hepato. 2006;18:143–50. doi: 10.1097/00042737-200602000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Irastorza I, Ibañez B, Delgado-Sanzonetti L, et al. Cow's-milk–free diet as a therapeutic option in childhood chronic constipation. J Pediatr Gastroenterol Nutr. 2010;51:171–6. doi: 10.1097/MPG.0b013e3181cd2653. [DOI] [PubMed] [Google Scholar]
- 3.Dehghani S-M, Ahmadpour B, Haghighat M, et al. The role of cow's milk allergy in pediatric chronic constipation: a randomized clinical trial. Iran J Pediatr. 2012;22:468. [PMC free article] [PubMed] [Google Scholar]
- 4.Iacono G, Carroccio A, Cavataio F, et al. Chronic constipation as a symptom of cow milk allergy. J Pediatr. 1995;126:34–9. doi: 10.1016/s0022-3476(95)70496-5. [DOI] [PubMed] [Google Scholar]
- 5.Sopo SM, Arena R, Greco M, et al. Constipation and cow's milk allergy: a review of the literature. Int Arch Allergy Immuno. 2014;164:40–5. doi: 10.1159/000362365. [DOI] [PubMed] [Google Scholar]
- 6.Sopo SM, Arena R, Scala G. Functional Constipation and Cow's-Milk Allergy. J Pediatr Gastroenterol Nutr. 2014;59:e34. doi: 10.1097/MPG.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 7.Syrigou EI, Pitsios C, Panagiotou I, et al. Food allergy-related paediatric constipation: the usefulness of atopy patch test. Eur J Pediatr. 2011;170:1173–8. doi: 10.1007/s00431-011-1417-6. [DOI] [PubMed] [Google Scholar]
- 8.Iacono G, Cavataio F, Montalto G, et al. Intolerance of cow's milk and chronic constipation in children. N Engl J Med. 1998;339:1100–4. doi: 10.1056/NEJM199810153391602. [DOI] [PubMed] [Google Scholar]
- 9.Simeone D, Miele E, Boccia G, et al. Prevalence of atopy in children with chronic constipation. Arch Dis Child. 2008;93:1044–47. doi: 10.1136/adc.2007.133512. [DOI] [PubMed] [Google Scholar]
- 10.van Ginkel R, Reitsma JB, Büller HA, Taminiau JA, Benninga MA. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology. 2003;125:357–63. doi: 10.1016/s0016-5085(03)00888-6. [DOI] [PubMed] [Google Scholar]
- 11.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroentero. 2011;25:3–18. doi: 10.1016/j.bpg.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Poli P, Scavuzzo F, Squassabia L, et al. Managing chronic functional constipation: Role of cow's milk allergy. Dig Liver Dis. 2016;48:245–6. [Google Scholar]
- 13.Tabbers M, DiLorenzo C, Berger M, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr J. 2014;58:258–74. doi: 10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 14.Brożek JL , Terracciano L , Hsu J , et al. Oral immunotherapy for IgE‐mediated cow's milk allergy: a systematic review and meta‐analysis. Clin Exp Allergy. 2012;42:363–74. doi: 10.1111/j.1365-2222.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 15.Carroccio A, Iacono G. CHRONIC CONSTIPATION AND FOOD ALLERGY. 2020. [Google Scholar]
- 16.Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol Hepatol. 2011;25:16–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Walker WA. Cow's milk protein-sensitive enteropathy at school age: a new entity or a spectrum of mucosal immune responses with age. J Pediatr. 2001;139:765–66. doi: 10.1067/mpd.2001.120265. [DOI] [PubMed] [Google Scholar]
- 18.El‐Hodhod M, Younis N, Zaitoun Y, Daoud S. Cow’s milk allergy related pediatric constipation: appropriate time of milk tolerance. Pediatr Allergy Immunol. 2010;21:407–12. doi: 10.1111/j.1399-3038.2009.00898.x. [DOI] [PubMed] [Google Scholar]
- 19.Inan M, Aydiner CY, Tokuc B, Aksu B, Ayvaz S, Ayhan S, et al. Factors associated with childhood constipation. J Paediatr Child Health. 2007;43(10):700–6. doi: 10.1111/j.1440-1754.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 20.Daher S, Tahan S, Solé D, Naspitz CK, Da Silva Patrício FR, Neto UF, et al. Cow's milk protein intolerance and chronic constipation in children. Pediatr Allergy Immunol. 2001;12:339–42. doi: 10.1034/j.1399-3038.2001.0o057.x. [DOI] [PubMed] [Google Scholar]
- 21.Andıran F, Dayı S, Mete E. Cow's milk consumption in constipation and anal fissure in infants and young children. J Paediatr Child Health. 2003;39:329–31. doi: 10.1046/j.1440-1754.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 22.Turunen S, Karttunen TJ, Kokkonen J. Lymphoid nodular hyperplasia and cow's milk hypersensitivity in children with chronic constipation. J Paediatr. 2004;145:606–1. doi: 10.1016/j.jpeds.2004.06.067. [DOI] [PubMed] [Google Scholar]