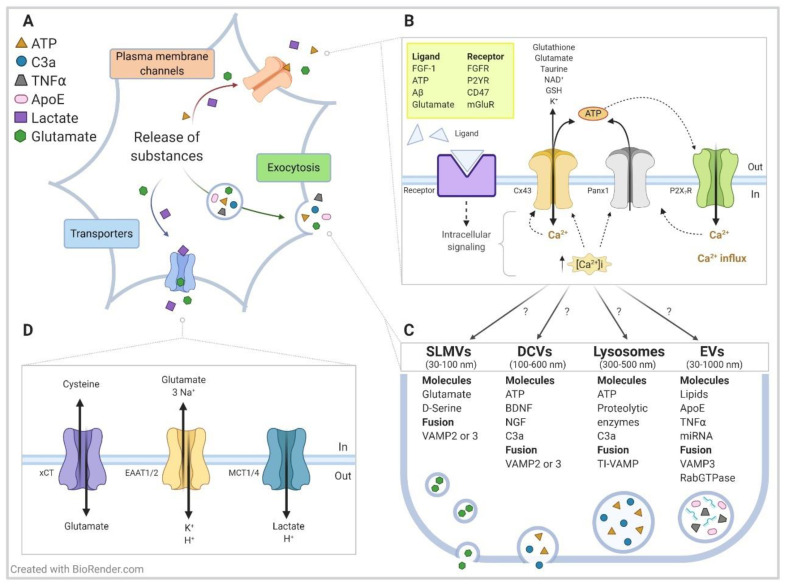
Figure 2.
Mechanisms of storage and release of bioactive substances in astrocytes: a focus on neurodegenerative diseases. Model showing (A) a general overview and (B–D) specific details of the release of signaling molecules from astrocytes into the extracellular space through three classical mechanisms. These mechanisms involve (B) diffusion through plasma membrane channels, (C) exocytosis, and (D) translocation by transporters. For simplicity, we show only a few membrane proteins, along with some bioactive substances and secretion signaling mechanisms that are critical for communicating with neurons and glial cells and are involved in diverse neurodegenerative diseases, particularly in Alzheimer´s disease and amyotrophic lateral sclerosis (ALS)/frontotemporal dementia (FTD). (B) Plasma membrane channels/receptors include HCs (mainly Cx43 and Panx1), purinergic receptors (e.g., P2XR, P2YR), growth factor receptors (e.g., fibroblast growth factor receptor (FGFR)) and ionic channels (e.g., VRAC, TREK-1; not shown). The influx of Ca2+ through HCs and ion channels—along with the initiation of intracellular signaling cascades through purinergic and growth factor receptors—promotes the release of Ca2+ from intracellular stores (predominantly endoplasmic reticulum) and increases cytoplasmic Ca2+ concentration ([Ca2+]i), which is a process that leads to the extracellular release of many bioactive substances by exocytosis. [Ca2+]i promotes the opening of Cx43 HCs and Panx HCs to directly release ATP into the extracellular environment. Cx43 HCs also release other small substances such as glutamate, NAD+, and glutathione. Extracellular Aβ also activates HCs. (C) Vesicles, such as SLMVs (synaptic-like microvesicles), DCVs (dense-core vesicles), lysosomes and EVs (extracellular vesicles, mainly microvesicles) contain diverse bioactive substances, including neurotransmitters, hormones and peptides, metabolic substrates, growth factors, and inflammatory factors, among others. Particular membrane fusion molecules (e.g., VAMP2 and TiVAMP) regulate the release of the vesicles. While many membrane proteins and bioactive substances are identified, little is known about the spatial and temporal aspects of vesicle release, including if membrane receptors/channels are localized in specific microdomains to trigger localized intracellular signaling pathways, and if elevated, [Ca2+]i promotes the fusion of specific vesicle types with a membrane that is adjacent either to neuronal synapses or away from the tripartite synapse. Understanding the mechanisms that underlie the differential release of vesicles would potentially open therapeutic avenues for constraining the secretion of critical toxic factors—such as glutamate, ATP, C3a, TNFα, apoliprotein E (ApoE), and certain miRNAs identified to play a relevant role in the pathogenesis of Alzheimer´s disease and ALS/FTD—without affecting the release of beneficial factors. Although less intensively studied, several of these toxic factors have been implicated in other neurodegenerative diseases. Of interest, a recent report shows that mutant huntingtin (mHtt) associates with Rab3a, which is a small GTPase localized on the membranes of DCVs, and it impairs brain-derived neurotrophic factor (BDNF) release from astrocytes. Another emerging topic is the potential role of connexons (mediated by Cx43 and Cx26) in recruiting molecules such as RNA and DNA into microvesicles and exosomes and the subsequent intercellular transfer of these vesicles. For simplicity, mHtt and connexons are not shown in the model. (D) The critical bioactive substances glutamate and lactate can also be released by transmembrane transporters such as the cystiene/glutamate antiporter (xCT), and monocarboxylate transporters (MCT1/4), respectively, independent of [Ca2+]i changes. The Na+-dependent glutamate excitatory amino acid transporters (EAAT1/2) are relevant for the uptake of extracellular glutamate and make a well-established contribution to excitotoxicity in ALS. For additional information on the release of bioactive substances and on the role of HCs in astrocytes, see [47,144,145,146,147]; on the Aβ-mediated opening of HCs, see [68]; on mHtt and impaired BDNF release from astrocytes, see [148]; and on connexons in the intercellular transfer of vesicles, see [149].