Table 1. Kinetic Parameters at pH 7.5 and 25 °C for Enzyme-Catalyzed Reactions of Whole and Phosphodianion Truncated Substrates (Scheme 2), the Total Phosphodianion Binding Energies, and the Dianion Binding Energy Utilized for Enzyme Activation.
| (kcat/Km)SPi | (kcat/Km)S | 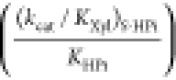 |
(ΔG⧧)Pi (kcal/mol),h,j | ΔGHPi⧧ (kcal/mol),i,j | ||
|---|---|---|---|---|---|---|
| enzyme | (M–1 s–1)e,f | (M–1 s–1)e,f | (M–2 s–1)f,g | [IBE]T | [IBE]HPi | [IBE]HPi/[IBE]T |
| ScPGIa | (6.9 ± 0.2) × 105 | (3.6 ± 0.2) × 10–4 | 0.52 ± 0.10 | 12.6 ± 0.1 | 4.3 ± 0.1 | 0.34 |
| kcat = 400 s–1 | ||||||
| LmG6PDHa | (2.0 ± 0.2) × 106 | (8.3 ± 0.1) × 10–3 | 53.0 ± 0.3 | 11.4 ± 0.1 | 5.2 ± 0.1 | 0.46 |
| kcat = 320 s–1 | ||||||
| Ec6PGDHb | (8.4 ± 0.4) × 105 | (9.9 ± 0.2) × 10–3 | 140 ± 1 | 10.8 ± 0.1 | 5.7 ± 0.1 | 0.53 |
| kcat = 12 s–1 | ||||||
| OMPDCc | 1.1 × 107 | 0.026 | 12000 | 11.7 ± 0.1 | 7.7 ± 0.1 | 0.66 |
| TIMd | 2.2 × 108 | 0.062 | 2700 | 13.0 ± 0.1 | 6.3 ± 0.1 | 0.48 |
| GPDHd | 4.6 × 106 | 0.050 | 16000 | 10.8 ± 0.1 | 7.5 ± 0.1 | 0.69 |
SPi = G6P; S = d-xylose.
SPi = 6-PG; S = d-xylonate.
Kinetic data for the catalyzed reactions of whole or truncated substrate (see SI).
The quoted uncertainty is the standard error obtained from the least-squares fit of experimental data to the appropriate kinetic equation.
Third-order rate constant for the phosphite dianion-activated reaction of truncated substrate (Scheme 3).
(ΔG⧧)Pi = RT ln[(kcat/Km)SPi/(kcat/Km)S].
The approximate uncertainties are calculated from the standard errors in the kinetic parameters.
