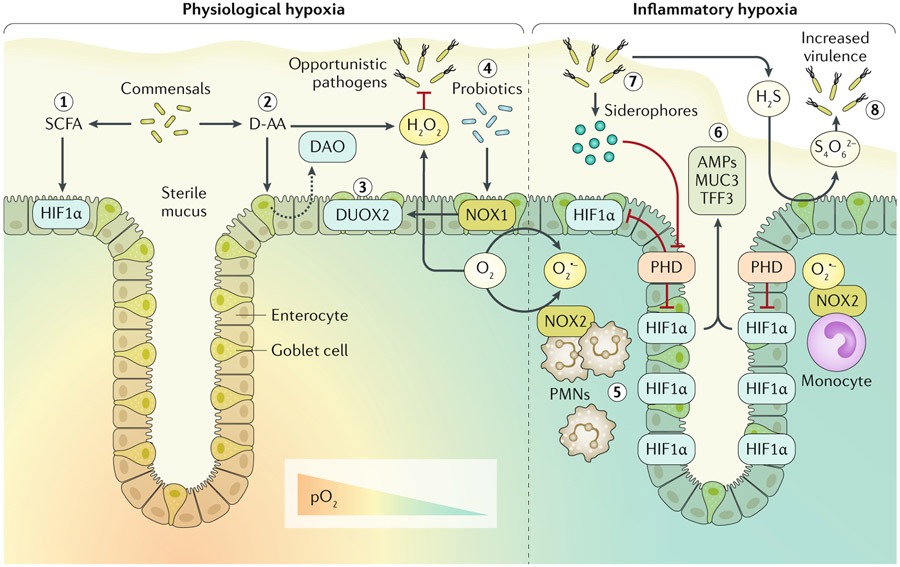
Fig. 1 ∣. Host-microbial redox signalling during hypoxia.
Enzymatic utilization of molecular oxygen (O2) within the intestinal mucosa facilitates redox signalling and results in both spatial and dynamic patterns of O2 availability. In the healthy intestinal mucosa, a steep O2 gradient exists between the highly vascularized mucosa and the anoxic lumen. Thus, cells within the crypt stem cell niche normally experience higher partial pressures of oxygen (pO2; ~100 mmHg) than the luminal-facing epithelia (<10 mmHg), which are known to normally experience hypoxia at homeostasis. This physiological hypoxia is experienced by epithelia adjacent to the lumen and results in stabilization of hypoxia-inducible factor (HIF). Gut microbiota secreting short chain fatty acids (SCFAs), particularly butyrate, contribute to this physiological hypoxia and associated stabilization of HIF1α through increased oxidative phosphorylation (step 1). Luminal redox signalling initiated by resident microorganisms releasing d-amino acids (D-AA) stimulates the epithelium to secrete D-AA oxidase (DAO) into the lumen, which subsequently yields hydrogen peroxide (H2O2) (step 2). Apical expression of epithelial dual oxidase 2 (DUOX2) probably results in luminal secretion of H2O2, which contributes to limiting opportunistic pathogen niche expansion (step 3). Probiotic lactobacilli upregulate epithelial NADPH oxidase 1 (NOX1) expression, which in turn induces DUOX2 (step4). Epithelial-expressed NOX1 and DUOX2, utilizing microenvironmental O2, generate oxygen radicals to further contribute to luminal release of H2O2. During inflammatory hypoxia, infiltrating polymorphonuclear leukocytes (PMNs) and monocytes expressing NOX2 generate superoxide (O2·−), resulting in inhibition of prolyl hydroxylase enzymes (PHD) and stabilization of HIF deep into the crypt (step 5). HIF transcriptional activity induces expression of barrier protective factors such as antimicrobial peptides (AMPs), mucin 3 (MUC3) and trefoil factor 3 (TFF3) (step 6). Certain opportunistic pathogens release siderophores, sequestering iron and inhibiting PHD (step 7). Sulfur metabolism of the mucosa can be hijacked by opportunistic pathogens. Hydrogen sulfide (H2S) is routinely detoxified to thiosulfate; however, high levels of reactive oxygen species within the mucosa can result in tetrathionate (S4O62−) generation, which can be utilized by Salmonella serotypes to provide a competitive advantage (step 8).