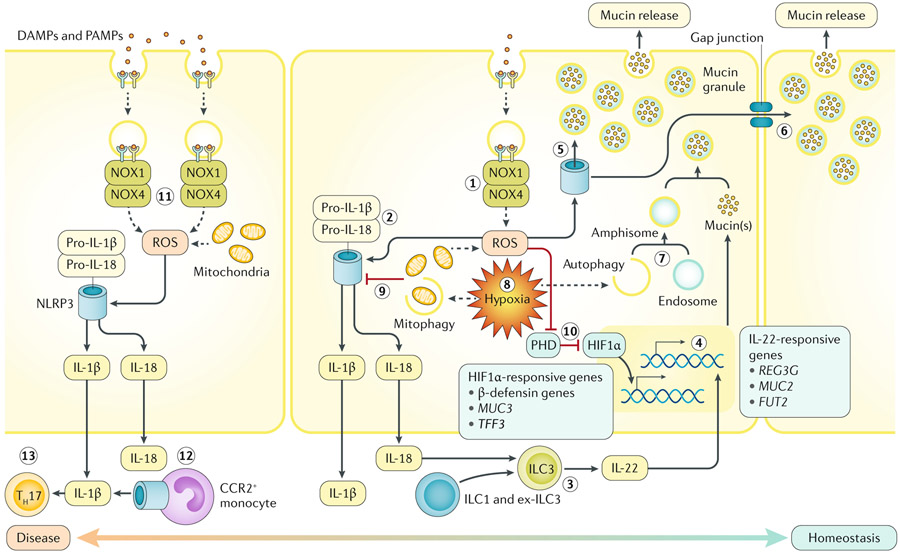
Fig. 2 ∣. Host redox–hypoxia crosstalk in the gastrointestinal mucosa.
The two major sources of endogenous reactive oxygen species (ROS) within the intestinal epithelium originate from mitochondria and NADPH oxidase 1 (NOX1) or NOX4 (step 1). In response to pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), epithelia recruit and activate the NOX1–NOX4 complex, stimulating superoxide and hydrogen peroxide generation (sources of ROS). Both enzymatic and mitochondria-derived ROS can trigger the activity of epithelial inflammasomes. In colonic epithelia, ROS-stimulated NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome assembly leads to IL-18 (and IL-1β) production (step 2). Although excessive mature secreted IL-18 is detrimental to epithelial integrity, the presence of IL-18 is necessary for IL-22 release by type 3 innate lymphoid cells (ILC3s) (step 3). ILC3-derived IL-22 promotes mucosal barrier protection by inducing mucin synthesis and goblet cell function (step 4). In goblet cells, ROS triggers the NLRP6 inflammasome to elicit mucin granule secretion (step 5). Sentinel goblet cells responding to microbial triggers can signal to adjacent goblet cells to degranulate via gap junctions (step 6). A combination of autophagic proteins, endosomes and NOX-derived ROS are necessary for mucin granule formation in goblet cells (step 7). Both autophagy and mitophagy are induced by hypoxia (step 8). Mitophagy might decrease NLRP3 inflammasome activity, reducing processing of IL-1β and IL-18 (step 9). Inhibition of prolyl hydroxylase enzymes (PHD) by ROS or hypoxia stabilizes hypoxia-inducible factor-1α (HIF1α), regulating barrier protective genes (step 10). Unimpeded or excessive ROS generation during active inflammation can lead to abundant maturation of IL-1β or IL-18 or even inflammasome-mediated cell death (necroptosis and pyroptosis) (step 11). Inflammasome-activation of infiltrating CC-chemokine receptor 2 (CCR2)+ monocytes contributes to active IL-1β (step 12). Mucosal IL-1β may lead to accumulation of IL-17A-secreting immune cells, such as T helper 17 cells (TH17) (step 13).