Figure 2. Active site architecture.
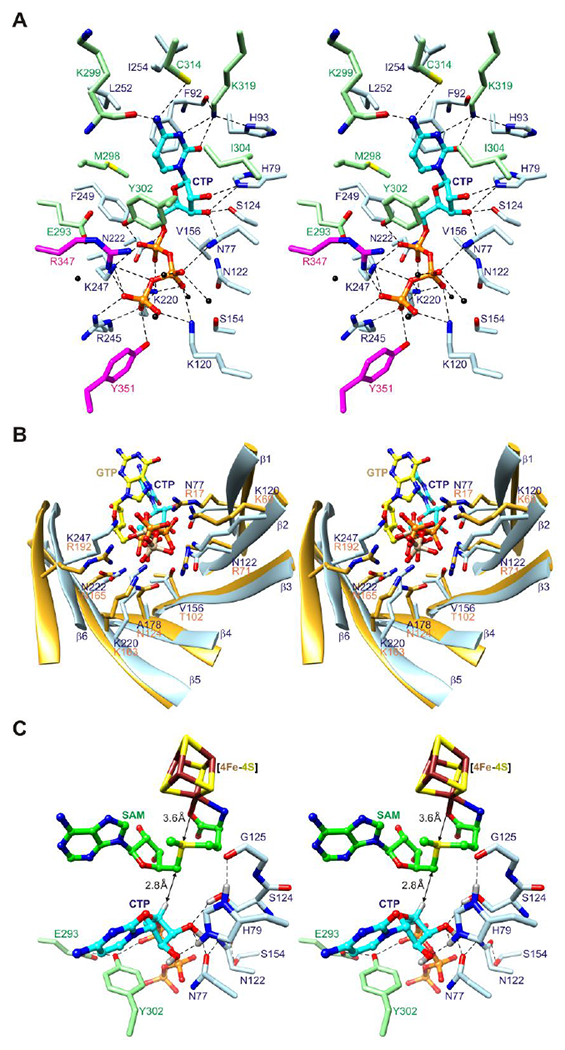
(A) Stereoview of CTP binding site. The side chains of twenty-five residues from the core domain and C-terminal extension, including two from the C-terminal tail (sticks), and five water molecules (black spheres) surround CTP (balls and sticks). Potential hydrogen bonds and other electrostatic interactions are shown as dashed lines. (B) Stereoview comparison of triphosphate binding residues of the (βα)6 partial barrels of viperin and MoaA. Eight residues in MoaA (PDB code 2FB333) bind the triphosphate moiety of GTP (balls and sticks with yellow colored carbon atoms and tan colored phosphorus atoms), five of which (Arg17, Lys69, Lys163, Asn165, and Arg192) have the same barrel locations as residues of viperin that bind the triphosphate moiety of CTP. (C) Stereoview model of substrate complex. SAM is modeled based on SAH coordinates.
