Significance Statement
Approximately 20% of patients with lupus nephritis, the most common renal manifestation of systemic lupus erythematosus, show membranous lupus nephritis on kidney biopsy, and nearly 10% eventually develop ESKD. Recently, two proteins, Exostosin 1 and Exostosin 2 (EXT1/EXT2), were shown to be present in a subset of membranous lupus nephritis kidney biopsy specimens. In an examination of 374 membranous lupus nephritis kidney biopsy specimens, the authors found 32.6% to be EXT1/EXT2-positive. Kidney biopsy specimens from patients with EXT1/EXT2-positive membranous lupus nephritis showed less chronicity features (glomerulosclerosis, interstitial fibrosis, and tubular atrophy) compared with those from EXT1/EXT2-negative patients. EXT1/EXT2-negative patients were also more likely to reach ESKD than EXT1/EXT2-positive patients. These findings suggest that the presence of EXT1/EXT2 is favorable, and that EXT1/EXT2-positive patients have better renal outcomes compared with EXT1/EXT2-negative patients.
Keywords: membranous nephropathy, renal biopsy, lupus nephritis
Abstract
Background
In patients with secondary (autoimmune) membranous nephropathy, two novel proteins, Exostosin 1 and Exostosin 2 (EXT1/EXT2), are potential disease antigens, biomarkers, or both. In this study, we validate the EXT1/EXT2 findings in a large cohort of membranous lupus nephritis.
Methods
We conducted a retrospective cohort study of patients with membranous lupus nephritis, and performed immunohistochemistry studies on the kidney biopsy specimens against EXT1 and EXT2. Clinicopathologic features and outcomes of EXT1/EXT2-positive versus EXT1/EXT2-negative patients were compared.
Results
Our study cohort included 374 biopsy-proven membranous lupus nephritis cases, of which 122 (32.6%) were EXT1/EXT2-positive and 252 (67.4%) were EXT1/EXT2-negative. EXT1/EXT2-positive patients were significantly younger (P=0.01), had significantly lower serum creatinine levels (P=0.02), were significantly more likely to present with proteinuria ≥3.5 g/24 h (P=0.009), and had significantly less chronicity features (glomerulosclerosis, P=0.001 or interstitial fibrosis and tubular atrophy, P<0.001) on kidney biopsy. Clinical follow-up data were available for 160 patients, of which 64 (40%) biopsy results were EXT1/EXT2-positive and 96 (60%) were EXT1/EXT2-negative. The proportion of patients with class 3/4 lupus nephritis coexisting with membranous lupus nephritis was not different between the EXT1/EXT2-positive and EXT1/EXT2-negative groups (25.0% versus 32.3%; P=0.32). The patients who were EXT1/EXT2-negative evolved to ESKD faster and more frequently compared with EXT1/EXT2-positive patients (18.8% versus 3.1%; P=0.003).
Conclusions
The prevalence of EXT1/EXT2 positivity was 32.6% in our cohort of membranous lupus nephritis. Compared with EXT1/EXT2-negative membranous lupus nephritis, EXT1/EXT2-positive disease appears to represent a subgroup with favorable kidney biopsy findings with respect to chronicity indices. Cases of membranous lupus nephritis that are EXT1/EXT2-negative are more likely to progress to ESKD compared with those that are EXT1/EXT2-positive.
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in White adults.1–3 Primary MN accounts for approximately 70%–80% of cases, and the remaining 20%–30% cases are secondary MN. In primary MN, M-Type Phospholipase A2 Receptor antigen (PLA2R), Thrombospondin Type-1 Domain-Containing 7A antigen (THSD7A), Neural EGF-like 1 protein (NELL1), and Semaphorin 3B (Sema3B) have been identified as target antigens.4–7 Secondary MN is associated with autoimmune diseases, malignancies, infection, and drugs.1 Recently, we discovered two novel proteins, Exostosin 1 and Exostosin 2 (EXT1/EXT2), in the glomerular basement membrane (GBM) in a small cohort of patients with PLA2R-negative MN.8,9 More than 80% of EXT1/EXT2-positive patients had an autoimmune finding, confirmed by serology, clinical presentation, or kidney biopsy findings of an underlying autoimmune disease.9
The discovery of EXT1/EXT2 proteins and their association with autoimmune diseases raises the possibility of a distinct subgroup of patients in the setting of lupus nephritis (LN). In this study, we aimed to analyze the following: (1) determine the prevalence of EXT1/EXT2 positivity in membranous (class 5) lupus nephritis (LMN); (2) determine whether EXT1/EXT2 positivity is exclusive to LMN or is present in LMN with proliferative features (class 3/4 LN); and (3) compare the clinicopathologic features and outcomes of EXT1/EXT2-positive and EXT1/EXT2-negative LMN.
Methods
Study Design
Using our institutional pathology database, patients with a diagnosis of LMN on kidney biopsy were screened from January 1, 2003 to December 30, 2018. The screening cohort included all biopsy specimens received in the Renal Pathology Laboratory at the Department of Laboratory Medicine and Pathology of the Mayo Clinic, for diagnosis and interpretation. The clinical information was obtained from the electronic medical records for the in-house biopsies. For other biopsy specimens received from outside institutions, clinical information was limited, and so medical records with pertinent clinical information were requested. The study was approved by the Mayo Clinic Institutional Review Board.
Patient Characteristics and Kidney Biopsy Data
Patients with clinical history of SLE and biopsy-proven LMN were further analyzed. Light microscopy, immunofluorescence microscopy, and electron microscopy were performed in each case. LN (classes 3 and 4) was classified according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification.10 All these patients had paraffin-embedded tissue blocks available. Immunohistochemistry (IHC) studies were performed on 4-μm tissue sections, using antibodies against EXT1/EXT2, as previously described.9 Laser microdissection and tandem mass spectrometry were performed on a subset of these cases. Details of the tandem mass spectrometry were recently described.6
Statistical Analyses
Categorical variables were presented as counts (percent) and continuous variables were presented as mean (SD), if they were normally distributed as determined by Shapiro-Wilk test, or as median (interquartile range [IQR]) if they were non-normal. For comparisons of categorical variables between groups, the Pearson chi-squared test was used if the number of elements in each cell was five or more; otherwise, the Fisher's exact test was used. For comparison of continuous variables between groups, an unpaired t test for independent samples was used for distributions consistent with normality as above, and the Mann–Whitney U test was used otherwise.
The Kaplan-Meier method was used to assess cumulative incidence of ESKD during the course of the follow-up. ESKD was defined as dependence on RRT. Cox proportional hazards regression models were used to determine predictive factors of the ESKD. We report the hazard ratios (HRs) with 95% confidence intervals (95% CIs), when appropriate.
P values <0.05 (two-sided) were considered significant. IBM SPSS Statistics for MacOS, version 25 (IBM, Armonk, NY), was used for data analysis.
Results
A total of 374 cases with adequate tissue for IHC were included in the study. All patients had a clinical history of SLE. The kidney biopsy specimens of all LMN cases showed the characteristic findings of thickened GBM on light microscopy, bright IgG and C3 staining along the capillary wall on immunofluorescence microscopy, and subepithelial deposits on electron microscopy.
Of 374 biopsy specimens, 122 (32.6%) had positive EXT1/EXT2 staining on IHC and 252 (67.4%) were negative for EXT1/EXT2 (Figure 1). IHC showed moderate to intense (2–3+/3) granular staining for both EXT1 and EXT2 along the GBM in the positive cases, and no staining in the negative cases. There were no intermediate cases of trace or minimal staining. EXT1 staining tends to be a little brighter when compared with EXT2 in the positive cases.
Figure 1.
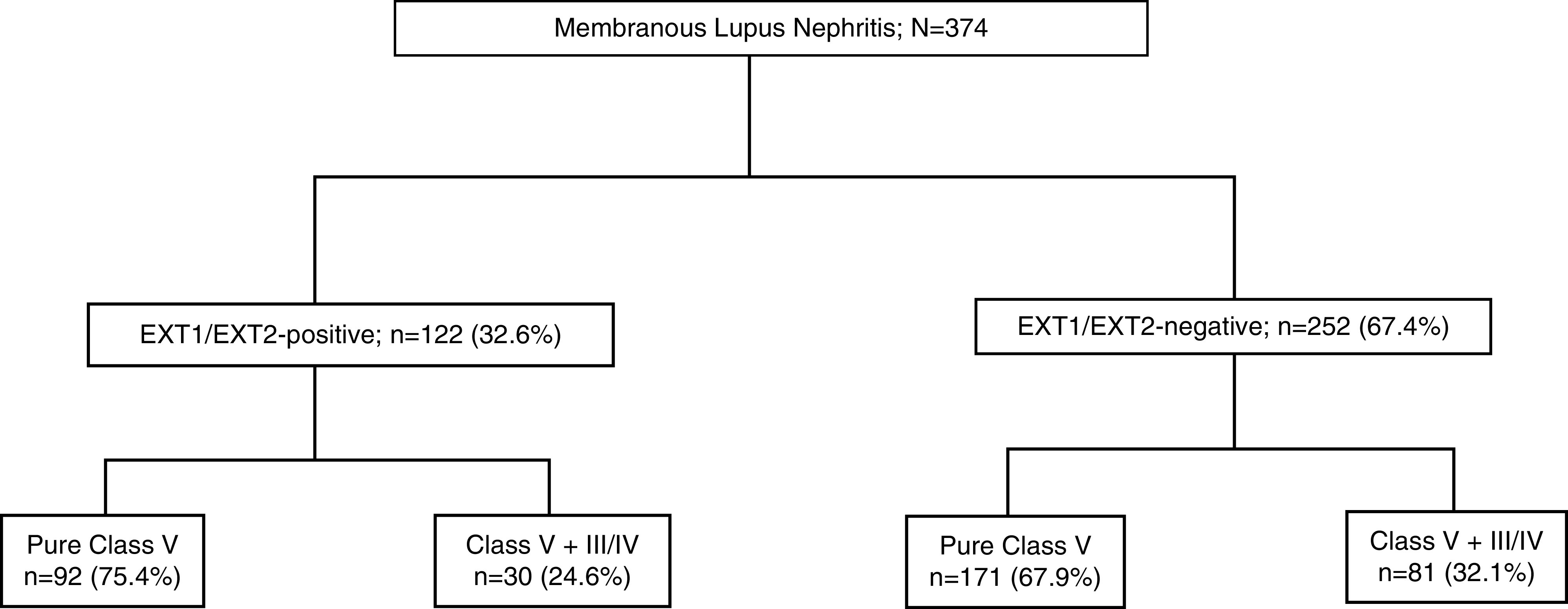
Study cohort of membranous lupus nephritis. Membranous lupus nephritisis categorized into EXT1/EXT2-positive and EXT1/EXT-2 negative cohorts, which are further subcategorized with and without proliferative features.
EXT1/EXT2-Positive LMN
Of the 122 patients, the median age at the time of kidney biopsy was 32 years (IQR, 25–41 years) (Table 1). The majority were female (n=106; 86.9%) patients. At presentation, the median serum creatinine (SCr) and proteinuria were 0.8 mg/dl (IQR, 0.6–1.2 mg/dl; n=112) and 3.5 g/24 h (IQR, 2–6.6 g/24 h; n=95), respectively. Fifty-seven (60%, n=95) patients presented with proteinuria ≥3.5 g/d at the time of kidney biopsy. Forty-one (33.6%) patients had hematuria.
Table 1.
Clinicopathologic features of EXT1/EXT2-positive and EXT1/EXT2-negative LMN
| Variable | EXT1/EXT2-Positive, n=122 | EXT1/EXT2-Negative, n=252 | P Valuea |
|---|---|---|---|
| Age, yr | Median: 32 | Median: 35 | 0.01 |
| IQR: 25–41 | IQR: 27–45 | ||
| Sex | Male: 16 (13.1%) | Male: 47 (18.7%) | 0.18 |
| Female: 106 (86.9%) | Female: 205 (81.3%) | ||
| SCr at presentation, mg/dl | Median: 0.8 | Median: 0.9 | 0.02 |
| IQR: 0.6–1.2; n=112 | IQR: 0.7–1.4; n=227 | ||
| Proteinuria at presentation, g/24 h | Median: 3.5 | Median: 3 | 0.08 |
| IQR: 2–6.6; n=95 | IQR: 1.8–4.8; n=199 | ||
| Proteinuria ≥3.5 g/d at presentation | 57 (60%); n=95 | 87 (43.7%); n=199 | 0.009 |
| Hematuria | 41 (33.6%) | 87 (34.5%) | 0.86 |
| Other autoimmune disease | 32 (26.2%) | 47 (18.7%) | 0.09 |
| Sclerosed glomeruli, % | Median: 3.8 | Median: 9.1 | 0.001 |
| IQR: 0–12.1 | IQR: 0–24.8 | ||
| IFTA, % | Median: 0 | Median: 10 | <0.001 |
| IQR: 0–10 | IQR: 5–20 | ||
| Proliferative features on light microscopy | 30 (24. 6%) | 81 (32.1%) | 0.13 |
| Immunofluorescence (glomerular staining) | Total cases with immunofluorescence (n=118) | Total cases with immunofluorescence (n=244) | — |
| IgG: 2–3+ (n=118, 100%) | IgG (n=244, 100%): | ||
| IgA (n=89, 75.4%); | trace (n=3); | ||
| trace (n=17); | 1+ (n=8); | ||
| 1+ (n=22); | 2–3+ (233) | ||
| 2–3+ (50) | IgA (n=186, 76.2%): | ||
| IgM (n=103, 87.3%): | trace (n=35); | ||
| trace (n=23); | 1+ (n=53); | ||
| 1+ (n=32); | 2–3+ (98) | ||
| 2–3+ (48) | IgM (n=220, 90.2%): | ||
| C1q (n=107, 90.7%): | trace (n=27); | ||
| trace (n=18); | 1+ (n=75); | ||
| 1+ (n=20); | 2–3+ (118) | ||
| 2–3+ (69) | C1q (n=216, 88.5%): | ||
| C3 (n=117, 99.2%): | trace (n=38); | ||
| trace (n=2); | 1+ (n=39); | ||
| 1+ (n=6); | 2–3+ (139) | ||
| 2–3+ (109) | C3 (n=238, 97.5%): | ||
| κ light chain (n=118, 100%): | trace (n=11); | ||
| trace (n=3); | 1+ (n=29); | ||
| 1+ (n=8); | 2–3+ (198) | ||
| 2–3+ (107) | κ light chain (n=241, 98.8%): | ||
| λ light chain (n=118, 100%): | trace (n=12); | ||
| trace (n=1); | 1+ (n=31); | ||
| 1+ (n=1); | 2–3+ (198) | ||
| 2–3+ (116) | λ light chain (n=241, 98.8%): | ||
| trace (n=10); | |||
| 1+ (n=22); | |||
| 2–3+ (209) | |||
| Electron microscopy | Subepithelial deposits: 122 (100%), of which 62 (50.8%) also had intramembranous deposits | Subepithelial deposits: 250 (99.2%), of which 153 (61.2%) also had intramembranous deposits | — |
| Mesangial/paramesangial deposits: 116 (95.1%) | Intramembranous deposits only: 2 (0.8%) | ||
| Subendothelial deposits: 71 (58.2%) | Mesangial/paramesangial deposits: 239 (94.8%) | ||
| Tubuloreticular inclusions: 107 (87.7%) | Subendothelial deposits: 146 (57.9%) | ||
| Tubuloreticular inclusions: 199 (79.0%) |
P<0.05 is considered significant.
Thirty-two (26.2%) patients had other associated autoimmune disorders, including Grave disease, antiphospholipid antibody syndrome, mixed connective tissue disease, rheumatoid arthritis, Sjögren syndrome, and CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia), and/or tested positive for other autoimmune serologies, including anti-Smith, anti-SSA, anti-SSB, anti-ribonucleoprotein, anti-nuclear cytoplasmic antibodies, anti–Scl-70, anti-histone and/or anti-chromatin antibodies, and lupus anticoagulant. Complement data were available in 62 patients, of which 31 (50%) had low C3 and C4, four (6.5%) had low C3 only, three (4.8%) had low C4 only, and the remaining 24 (38.7%) had normal complement levels.
Ninety-two (75.4%) biopsy specimens showed pure class 5 LMN, and the remaining 30 (24.6%) biopsy specimens had mixed class 5 LMN with proliferative features (19 class 3 and 11 class 4 LN; Table 1). The kidney biopsy specimens of all cases of EXT1/EXT2-positive LMN showed the characteristic findings of thickened GBM on light microscopy (Figure 2). Overall, on average, 16.6±10.0 glomeruli were present, with a median of 3.8% global glomerulosclerosis (IQR, 0%–12.1%). Interstitial fibrosis and tubular atrophy (IFTA) was minimal (<10%) in 89 (73%) cases, mild (10%–25%) in 21 (17.2%) cases, moderate (26%–50%) in eight (6.6%) cases, and severe (>50%) in four (3.3%) cases. Overall, the median IFTA was 0% (IQR, 0%–10%). Immunofluorescence studies were performed in 118 of 122 biopsy specimens where sufficient glomeruli were present for evaluation. Of these 118 biopsy specimens, all demonstrated bright IgG (2–3+/3), 89 showed IgA (trace–3+/3), 103 showed IgM (trace–3+/3), 118 showed κ and λ light chains (trace–3+/3), 107 showed C1q (trace–3+/3), and 117 showed C3 (trace–3+/3). Immunofluorescence study for PLA2R was evaluated in ten cases and all were negative. On electron microscopy, eight (6.6%) cases were class 1, 58 (47.5%) cases were class 2, 56 cases (45.9%) were class 3, and none of the cases were class 4, on the basis of the Ehrenreich and Churg classification (Table 2). Subendothelial deposits and mesangial/paramesangial deposits were present in 71 (58.2%) and 116 (95.1%) of 122 cases, respectively. Tubuloreticular inclusions were present in the endothelial cells in 107 (87.7%) cases. Laser microdissection and tandem mass spectrometry was performed in a subset of cases (n=8) that showed high spectral counts for EXT1 (average, 88.6±37.2) and EXT2 (average, 66.1±34.6), thus confirming the IHC findings (data not shown).
Figure 2.
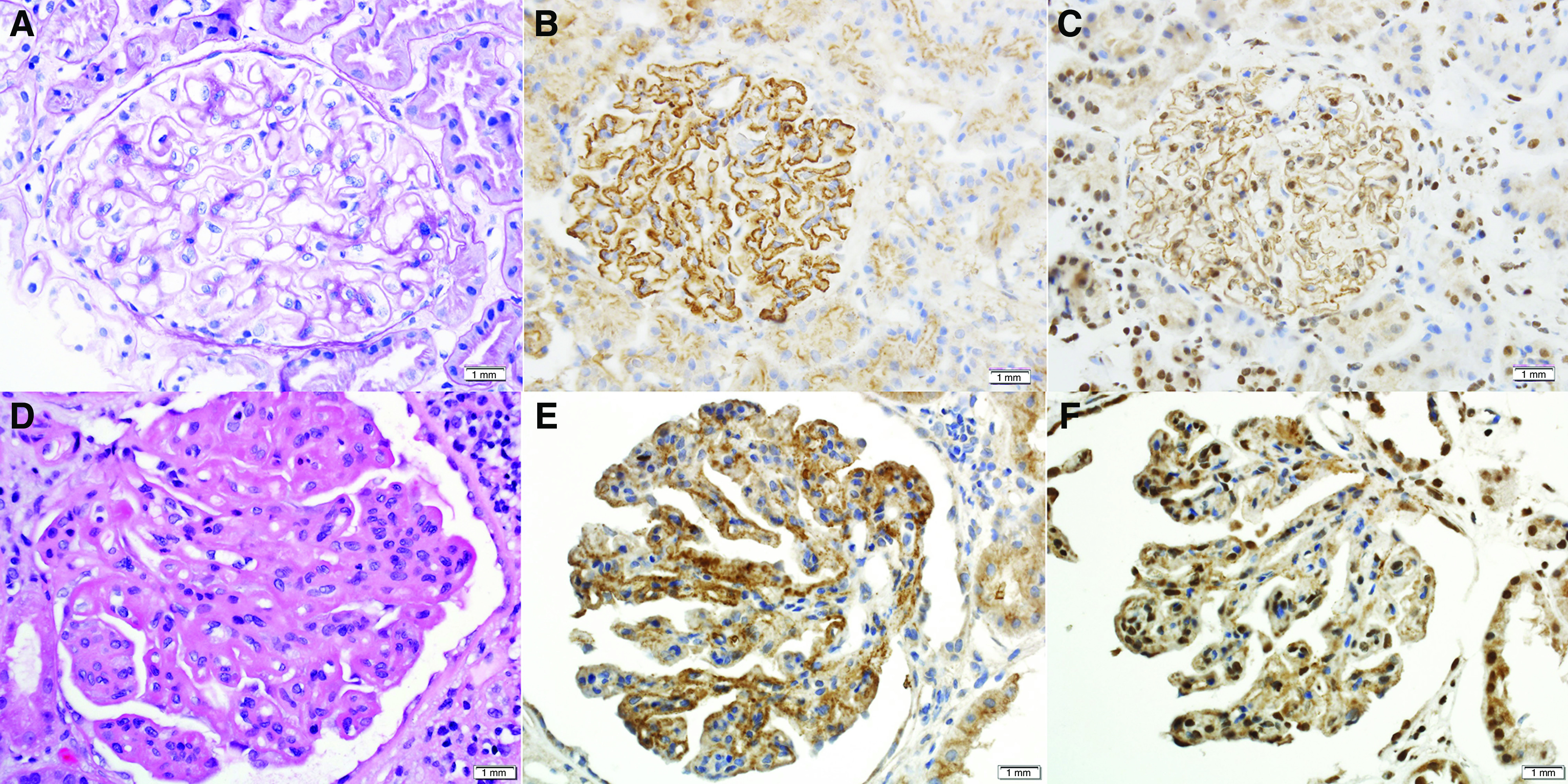
Light microscopy and immunohistochemistry (IHC) of EXT1/EXT2-positive LMN.(A–C) Top panel showing pure class 5 LMN, and (D–F) bottom panel showing class 5 LMN with class 3 proliferative LN. (B and E) IHC for EXT1 (×40 magnification). (C and F) IHC for EXT2 (×40 magnification).
Table 2.
Electron microscopy class on the basis of the Ehrenreich and Churg classification
| Ehrenreich and Churg Classification | EXT1/EXT2-Negative, n=252 (67.4%) | EXT1/EXT2-Positive, n=122 (32.6%) | P Valuea |
|---|---|---|---|
| 1 | 9 (3.6%) | 8 (6.6%) | 0.19 |
| 2 | 72 (28.6%) | 58 (47.5%) | <0.001 |
| 3 | 167 (66.3%) | 56 (45.9%) | <0.001 |
| 4 | 3 (1.2%) | 0 (0.0%) | 0.23 |
P<0.05 is considered significant.
EXT1/EXT2-Negative LMN
Of the 252 patients, the median age at the time of kidney biopsy was 35 years (IQR, 27–45 years) (Table 1). The majority were female (n=205; 81.3%). At the time of kidney biopsy, the median SCr and urine proteinuria were 0.9 mg/dl (IQR, 0.7–1.4 mg/dl; n=227) and 3 g/24 h (IQR, 1.8–4.8 g/24 h; n=199), respectively. Eighty-seven (43.7%, n=199) patients presented with proteinuria ≥3.5 g/d at the time of kidney biopsy. Eighty-seven (34.5%) patients had hematuria.
Forty-seven (18.7%) patients had other associated autoimmune disorders (rheumatoid arthritis, Sjögren syndrome, Grave disease, mixed connective tissue disease, immune thrombocytopenic purpura) and/or tested positive for other autoimmune serologies, including anti-Smith, rheumatoid factor, anti-SSA, anti-SSB, anti-ribonucleoprotein, anti-nuclear cytoplasmic antibodies, anti–Scl-70, and anti-histone antibodies. Complement data were available in 126 patients, of which 62 (49.2%) had low C3 and C4, 20 (15.9%) had low C3 only, 11 (8.7%) had low C4 only, and the remaining 33 (26.2%) had normal complement levels.
A total of 171 (67.9%) cases showed pure class 5 LMN and remaining 81 (32.1%) cases had mixed class 5 LMN with proliferative features (29 had class 3 LN and 52 had class 4 LN). The kidney biopsy specimens of all cases of EXT1/EXT2-negative LMN showed the characteristic findings of thickened GBM on light microscopy (Figure 3). Overall, an average of 19.3±11 glomeruli were present, with a median of 9.1% global glomerulosclerosis (IQR, 0%–24.8%). IFTA was minimal (<10%) in 120 (47.6%) cases, mild (10%–25%) in 91 (36.1%) cases, moderate (26%–50%) in 34 (13.5%) cases, and severe (>50%) in seven (2.8%) cases. Overall, the median IFTA was 10% (IQR, 5%–20%). Immunofluorescence studies were performed in 244 of 252 biopsy specimens where sufficient glomeruli were present for evaluation. Of these 244 biopsy specimens, all demonstrated IgG, 238 showed C3 (trace–3+/3), 216 showed C1q (trace–3+/3), 220 showed IgM (trace–3+/3), 186 showed IgA (trace–3+/3), and 241 showed κ and λ light chains (trace–3+/3). Immunofluorescence study for PLA2R was evaluated in ten cases and all were negative. On electron microscopy, nine (3.6%) cases were class 1, 72 (28.6%) cases were class 2, 167 cases (66.3%) were class 3, and three (1.2%) cases were class 4, on the basis of the Ehrenreich and Churg classification (Table 2). Subendothelial and mesangial/paramesangial deposits were present in 146 (57.9%) and 239 (94.8%) of 252 cases, respectively. Tubuloreticular inclusions were present in 199 (79%) cases. Laser microdissection and tandem mass spectrometry was performed in a subset of cases (n=7), and were negative for EXT 1 and EXT2, thus confirming the IHC findings (data not shown).
Figure 3.
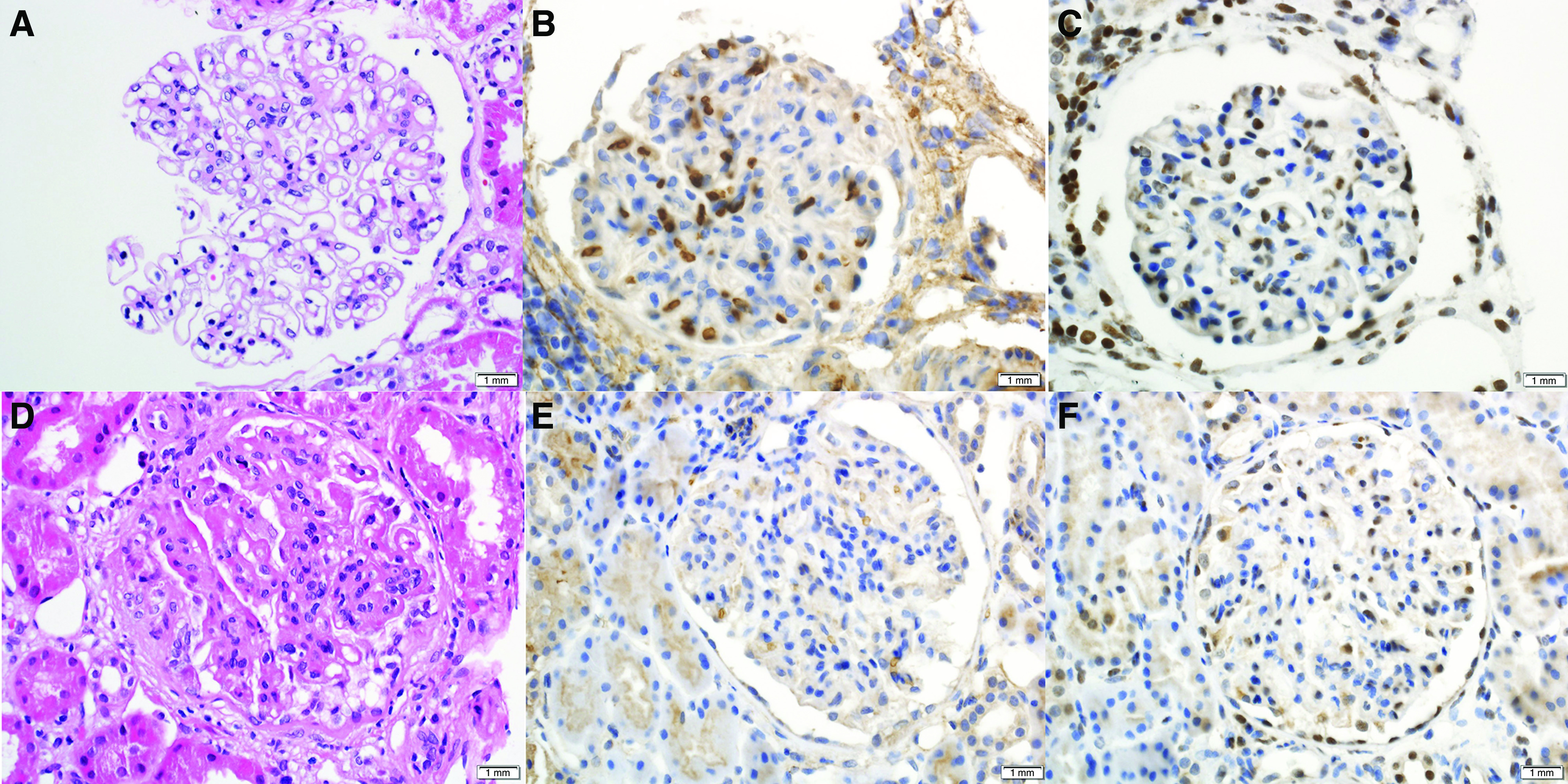
Light microscopy and immunohistochemistry (IHC) of EXT1/EXT2-negative LMN. (A–C) Top panel showing pure class 5 LMN, (D–F) and bottom panel showing class 5 LMN with class 3 proliferative LN. (B and E) IHC for EXT1 (×40 magnification). (C and F) IHC for EXT2 (×40 magnification).
The clinical and laboratory features comparing patients with EXT1/EXT2-positive and EXT1/EXT-2 negative LMN are described in Table 1. In summary, compared with the EXT1/EXT2-negative cohort (n=252), the EXT1/EXT2-positive LMN (n=122) cohort were slightly younger (P=0.01), have lower SCr levels (P=0.02), tend to present with proteinuria ≥3.5 g/24 h (P=0.009), and have less chronicity (glomerulosclerosis: P=0.001; IFTA: P<0.001). On the basis of the Ehrenreich and Churg classification, a higher proportion of EXT1/EXT2-negative LMN have class 3 lesions compared with EXT1/EXT2-positive LMN (66.3% versus 45.9%; P<0.001). On the other hand, a higher proportion of EXT1/EXT2-positive LMN have class 2 lesions compared with EXT1/EXT2-negative LMN (47.5% versus 28.6%; P<0.001).
EXT1/EXT2 in Proliferative LN without LMN
We also stained 38 cases of class 1–4 LN (class 1: n=1; class 2: n=7; class 3: n=6; class 4; n=24) with no component of LMN. All cases were negative for EXT1/EXT2 on IHC.
Rebiopsy of LMN
During the course of follow-up, four LMN cases had rebiopsy after the initial diagnosis of LMN; three were EXT1/EXT2-positive and one was EXT1/EXT2-negative LMN. All three biopsy specimen of EXT-positive LMN did not show significant progression of chronicity indices, whereas the single EXT1/EXT2-negative LMN biopsy specimen showed increased global glomerulosclerosis and IFTA (Table 3). EXT1 staining was done in rebiopsies of four cases, and showed bright (3+) EXT1 staining in two cases (case 1 and 2) and weak (1+) staining in one case (case 3) of EXT1/EXT2-positive LMN; it was negative in one case of EXT1/EXT2-negative LMN. In two EXT1/EXT2-positive LMN cases (case 1 and 2), rebiopsies were done after 2 years of follow-up; in one EXT1/EXT2-positive LMN case, rebiopsy was done after 10 years of follow-up; and in the one EXT1/EXT2-negative LMN case, rebiopsy was done after 7 years of follow-up.
Table 3.
Chronicity findings in LMN on rebiopsy
| Case | Glomeruli/Sclerosed | IFTA, % | Follow-Up, yr | Rebiopsy: Glomeruli/Sclerosed | Rebiopsy: IFTA, % |
|---|---|---|---|---|---|
| 1 | 17/1 (5.9%) | 0 | 2 | 17/2 (11.8%) | 0 |
| 2 | 10/0 (0%) | 0 | 2 | 26/0 (0%) | 0 |
| 3 | 3/0 (0%) | 0 | 10 | 42/2 (4.8%) | 0 |
| 4 | 7/0 (0%) | 0 | 7 | 42/19 (45.2%) | 25 |
Cases 1–3 are EXT1/EXT2-positive LMN and case 4 is EXT1/EXT2-negative LMN.
Clinical Follow-Up of EXT1/EXT2-Positive and EXT-Negative LMN
Clinical follow-up data were available in a total of 160 patients. Clinical characteristics and follow-up and outcome data are summarized in Figure 4 and Tables 4–6.
Figure 4.
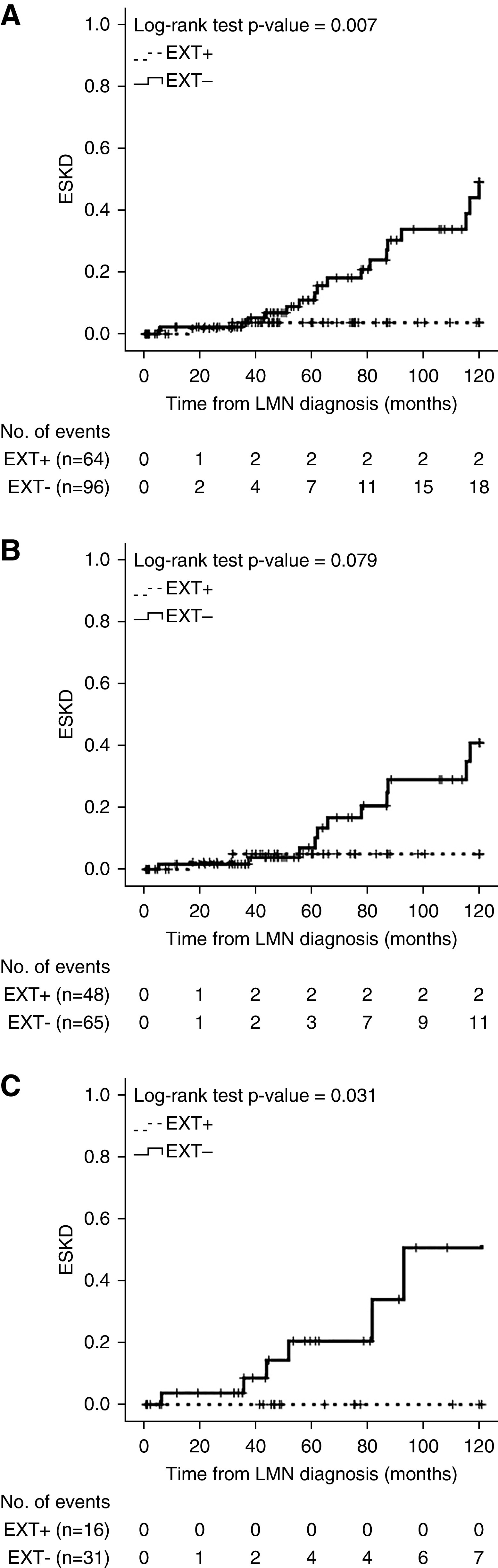
Cumulative incidence of ESKD in patients with LMN.Kaplan-Meier plots of the cumulative incidence of ESKD over 10 years. (A) EXT1/EXT2-positive and EXT1/EXT2-negative LMN (with or without class 3/4 LN): 64 EXT1/EXT2-positive versus 96 EXT1/EXT2-negative LMN; two versus 18 events; P=0.007. (B) EXT1/EXT2-positive and EXT1/EXT2-negative pure class 5 LMN (with no class 3/4 LN): 48 EXT1/EXT2-positive versus 65 EXT1/EXT2-negative LMN; two versus 11 events; P=0.08. (C) EXT1/EXT2-positive and EXT1/EXT2-negative class 5 LMN with class 3/4 LN: 16 EXT1/EXT2-positive versus 31 EXT1/EXT2-negative LMN; zero versus seven events; P=0.03.
Table 4.
Clinical characteristics, follow-up, and outcomes of EXT1/EXT2-positive and EXT1/EXT2-negative LMN, with and without LN (combined class 5 LMN with or without class 3/4 LN)
| Variable | EXT1/EXT2-Positive LMN, n=64/122 (52.5%) | EXT1/EXT2-Negative LMN, n=96/252 (38.1%) | P Valuea |
|---|---|---|---|
| Age at presentation, median (IQR), yr | 33 (25–42) | 38 (28–47) | 0.10 |
| Female, n (%) | 57 (89.1) | 75 (78.1) | 0.07 |
| SCr at presentation, median (IQR), mg/dl | 0.80 (0.60–1.00), n=61 | 0.90 (0.70–1.40), n=89 | 0.004 |
| Proteinuria at presentation, median (IQR), g/24 h | 3.9 (1.6–6.7) n=53 | 3.0 (1.5–4.9), n=76 | 0.3 |
| Proteinuria at presentation ≥3.5/d, n (%) | 32 (60.4), n=53 | 33 (43.4), n=76 | 0.06 |
| Hematuria, n (%) | 19 (29.7) | 35 (36.5) | 0.38 |
| Proliferative features, n (%) | 16 (25.0) | 31 (32.3) | 0.32 |
| Other autoimmune diseases, n (%) | 19 (29.7) | 22 (22.9) | 0.34 |
| Sclerosed glomeruli, median (IQR), % | 0.0 (0.0–10.2) | 13.2 (1.82–25.0) | <0.001 |
| IFTA, median (IQR), % | 0.0 (0.0–0.0) | 10.0 (5.0–20.0) | <0.001 |
| SCr at end of follow-up, median (IQR), mg/dl | 0.90 (0.70–1.20), n=51 | 1.10 (0.70–1.60), n=71 | 0.05 |
| Proteinuria at end of follow-up, median (IQR), g/24 h | 0.85 (0.20–2.00), n=36 | 0.90 (0.25–2.35), n=57 | 0.89 |
| Proteinuria at end of follow-up ≥3.5/d, n (%) | 3 (8.3), n=36 | 9 (15.8), n=57 | 0.3 |
| ESKD, n (%) | 2 (3.1) | 18 (18.8) | 0.003 |
| Death, n (%) | 2 (3.1) | 8 (8.3) | 0.18 |
| Time of follow-up, median (IQR), mo | 48.6 (33.1–76.5) | 50.6 (32.7–86.8) | 0.85 |
P<0.05 is considered significant.
Table 5.
Clinical characteristics, follow-up, and outcomes of EXT1/EXT2-positive and EXT1/EXT2-negative LMN without proliferative features (pure class 5)
| Variable | EXT1/EXT2-Positive LMN, n=48/64 (75.0%) | EXT1/EXT2-Negative LMN, n=65/96 (67.7%) | P Valuea |
|---|---|---|---|
| Age at presentation, median (IQR), yr | 33 (25–42) | 40 (28–47) | 0.12 |
| Female, n (%) | 42 (87.5) | 53 (81.5) | 0.39 |
| SCr at presentation, median (IQR), mg/dl | 0.70 (0.60–0.90), n=46 | 0.80 (0.60–1.30), n=58 | 0.01 |
| Proteinuria at presentation, median (IQR), g/24 h | 3.5 (1.5–5.9), n=40 | 2.8 (1.40–4.3), n=51 | 0.23 |
| Proteinuria at presentation ≥3.5/d, n (%) | 23 (57.5), n=40 | 18 (35.3), n=51 | 0.04 |
| Hematuria, n (%) | 15 (31.3) | 22 (33.8) | 0.77 |
| Other autoimmune diseases, n (%) | 14 (29.2) | 15 (23.1) | 0.46 |
| Sclerosed glomeruli, median (IQR), % | 0.0 (0.0–8.5) | 9.5 (0.0–24.4) | <0.001 |
| IFTA, median (IQR), % | 0.0 (0.0–0.0) | 10.0 (5.0–25.0) | <0.001 |
| SCr at end of follow-up, median (IQR), mg/dl | 0.85 (0.70–1.13), n=38 | 1.00 (0.70–1.57), n=51 | 0.09 |
| Proteinuria at end of follow-up, median (IQR), g/24 h | 0.95 (0.20–2.55), n=26 | 0.90 (0.20–1.93), n=42 | 0.46 |
| Proteinuria at end of follow-up ≥3.5/d, n (%) | 3 (11.5), n=26 | 6 (14.3), n=42 | 0.75 |
| ESKD, n (%) | 2 (4.2) | 11 (16.9) | 0.04 |
| Death, n (%) | 2 (4.2) | 2 (3.1) | 0.76 |
| Time of follow-up, median (IQR), mo | 52.5 (31.6–81.2) | 53.6 (34.2–97.1) | 0.61 |
P<0.05 is considered significant.
Table 6.
Clinical characteristics, follow-up, and outcomes of all EXT1/EXT2-positive and EXT1/EXT2-negative LMN with proliferative features (class 5 with class 3/4 LN)
| Variable | EXT1/EXT2-Positive LMN, n=16/64 (25.0%) | EXT1/EXT2-Negative LMN, n=31/96 (32.3%) | P Valuea |
|---|---|---|---|
| Age at presentation, median (IQR), yr | 32 (25–43) | 33 (28–47) | 0.39 |
| Female, n (%) | 15 (93.8) | 22 (71.0) | 0.07 |
| SCr at presentation, median (IQR), mg/dl | 1.0 (0.8–2.0), n=15 | 1.20 (0.8–2.3), n=31 | 0.35 |
| Proteinuria at presentation, median (IQR), g/24 h | 6.6 (1.5–7.9), n=13 | 3.7 (1.7–6.5), n=25 | 0.48 |
| Proteinuria at presentation ≥3.5/d, n (%) | 9 (69.2), n=13 | 15 (60), n=25 | 0.58 |
| Hematuria, n (%) | 4 (25.0) | 13 (41.9) | 0.25 |
| Other autoimmune diseases, n (%) | 5 (31.3) | 7 (22.6) | 0.52 |
| Sclerosed glomeruli, median (IQR), % | 4.6 (0.0–12.5) | 20.0 (3.22–27.8) | 0.01 |
| IFTA, median (IQR), % | 0.0 (0.0–8.75) | 10.0 (5.0–20.0) | 0.003 |
| SCr at end of follow-up, median (IQR), mg/dl | 0.90 (0.8–1.5), n=13 | 1.30 (0.70–1.9), n=20 | 0.32 |
| Proteinuria at end of follow-up, median (IQR), g/24 h | 0.60 (0.20–1.25), n=10 | 1.5 (0.30–3.40), n=15 | 0.12 |
| Proteinuria at end of follow-up ≥3.5/d, n (%) | 0 (0.0), n=10 | 3 (20.0), n=15 | 0.13 |
| ESKD, n (%) | 0 (0.0) | 7 (22.6) | 0.04 |
| Death, n (%) | 0 (0.0) | 6 (19.4) | 0.06 |
| Time of follow-up, median (IQR), mo | 48.6 (43.0–76.3) | 43.4 (27.1–77.9) | 0.27 |
P<0.05 is considered significant.
Combined Class 5 LMN with or without Class 3/4 LN
Of the 160 patients with follow-up data, 64 (40%) were EXT1/EXT2-positive and 96 (60%) were EXT1/EXT2-negative LMN (Table 4). The proportion of patients with proliferative features was not statistically significantly different between groups: 16 (25%) of 64 patients in the EXT1/EXT2-positive group versus 31 (32.3%) of 96 patients in the EXT1/EXT2-negative group showed proliferative features (P=0.32). At presentation, the median SCr was higher in the EXT1/EXT2-negative group (0.90 versus 0.80 mg/dl; P=0.004), but there were no differences in the presence of hematuria, or proteinuria ≥3.5 g/d. Kidney biopsy specimens of EXT1/EXT2-negative patients showed a significantly higher median of global glomerulosclerosis and IFTA (P<0.001). The treatment was variable and included the following: (1) EXT1/EXT2-positive LMN: five received steroids only, 14 received immunosuppressants, and 22 received a combination of steroids and immunosuppressants, and treatment details were not available for the remaining patients; (2) EXT1/EXT2-negative LMN: three were managed conservatively, four received steroids only, 18 received immunosuppressants, and 51 received a combination of steroids and immunosuppressants, and treatment details were not available for the remaining patients. The most common immunosuppressants included plaquenil, mycophenolate mofetil, cyclophosphamide, and azathioprine.
Both groups were followed for a similar period of time (48.6 versus 50.6 months; P=0.85). At the end of the follow-up period, patients who were EXT1/EXT2-negative presented with higher values of SCr (1.10 versus 0.90 mg/dl; P=0.05). During the course of the disease, patients who were EXT1/EXT2-negative evolved to ESKD more frequently (18.8% versus 3.1%; P=0.003), and the time to this event was significantly shorter in this group when compared with EXT1/EXT2-positive patients (101 versus 116 months; P=0.007) ( Figure 4A). There was no difference in death rates between groups. Using multivariable Cox regression, we determined that the predictive factors for the development of ESKD at 10 years were male sex (HR, 2.92; 95% CI, 1.16 to 7.34; P=0.02) and glomerulosclerosis ≥25% (HR, 4.03; 95% CI, 1.67 to 19.71; P=0.002) (Table 7).
Table 7.
Multivariable analysis of predictive factors for ESKD at 10 years
| Factors | Univariable Cox Regression | Multivariable Cox Regression | ||
|---|---|---|---|---|
| HR (95% CI) | P Valuea | HR (95% CI) | P Valuea | |
| Combined class 5 LMN with or without class 3/4 LN | ||||
| Age | 0.97 (0.94 to 1.01) | 0.21 | ||
| Male | 2.82 (1.12 to 7.08) | 0.03 | 2.92 (1.16 to 7.34) | 0.02 |
| EXT1/EXT2-negative | 5.85 (1.36 to 25.24) | 0.02 | ||
| SCr at presentation | 1.36 (1.10 to 1.69) | 0.004 | ||
| Proteinuria at presentation | 0.96 (0.83 to 1.11) | 0.58 | ||
| Hematuria | 1.15 (0.66 to 1.98) | 0.63 | ||
| Other autoimmune diseases | 1.08 (0.39 to 2.97) | 0.88 | ||
| ≥25% glomerulosclerosis | 3.93 (1.63 to 9.47) | 0.002 | 4.03 (1.67 to 19.71) | 0.002 |
| ≥25% IFTA | 4.23 (1.74 to 10.28) | 0.001 | ||
| Only class 5 LMN (without class 3/4 LN) | ||||
| Age | 0.99 (0.95 to 1.04) | 0.67 | ||
| Male | 2.35 (0.72 to 7.70) | 0.16 | ||
| EXT1/EXT2-negative | 3.56 (0.79 to 16.08) | 0.1 | ||
| SCr at presentation | 1.27 (0.9 to 1.81) | 0.18 | ||
| Proteinuria at presentation | 1.02 (0.91 to 1.15) | 0.77 | ||
| Hematuria | 1.40 (0.66 to 2.99) | 0.38 | ||
| Other autoimmune diseases | 1.29 (0.4 to 4.18) | 0.68 | ||
| ≥25% glomerulosclerosis | 4.31 (1.44 to 12.88) | 0.009 | ||
| ≥25% IFTA | 3.91 (1.31 to 11.73) | 0.02 | ||
| Class 5 LMN with class 3/4 LN | ||||
| Age | 0.93 (0.84 to 1.04) | 0.20 | ||
| Male | 6.33 (1.03 to 38.8) | 0.05 | 8.35 (1.11 to 62.60) | 0.04 |
| EXT1/EXT2-negative | — | — | — | — |
| SCr at presentation | 1.40 (1.02 to 1.92) | 0.04 | 1.53 (1.04 to 2.25) | 0.03 |
| Proteinuria at presentation | 0.21 (0.02 to 3.04) | 0.25 | ||
| Hematuria | 0.73 (0.30 to 1.75) | 0.48 | ||
| Other autoimmune diseases | 0.85 (0.1 to 7.50) | 0.88 | ||
| ≥25% glomerulosclerosis | 2.52 (0.55 to 11.50) | 2.52 | ||
| ≥25% IFTA | 6.36 (1.27 to 31.80) | 0.02 | ||
P<0.05 is considered significant.
Only Class 5 LMN without Class 3/4 LN
Forty-eight (75%) of 64 EXT1/EXT2-positive cases and 65 (67.7%) of 96 EXT1/EXT2-negative cases had pure class 5 with no class 3/4 LN (Table 5). Similarly to what was observed in the combined cohort, EXT1/EXT2-negative cases had higher values of SCr at diagnosis when compared with EXT1/EXT2-positive patients (0.80 versus 0.70 mg/dl; P=0.01). The EXT1/EXT2 positive patients presented more frequently with proteinuria ≥3.5 g/d at diagnosis (57.5% versus 35.3%; P=0.04). Kidney biopsy specimens of EXT1/EXT2-negative patients showed a significantly higher median of global glomerulosclerosis and IFTA (P<0.001). At the end of the follow-up, there were no differences between groups on the median SCr or proteinuria ≥3.5 g/d. During the course of the disease, patients who were EXT1/EXT2-negative evolved to ESKD more frequently (16.9% versus 4.2%; P=0.04), but the mean time to ESKD was not significantly different between groups (104 versus 115 months; P=0.08) (Figure 4B). Using univariable Cox regression, we determined that the predictive factors for the development of ESKD at 10 years were glomerulosclerosis ≥25% (HR, 4.31; 95% CI, 1.44 to 12.88; P=0.009) and IFTA ≥25% (HR, 3.91; 95% CI, 1.31 to 11.73; P=0.02) (Table 7).
Class 5 LMN with Class 3/4 LN
Sixteen (25%) of 64 EXT1/EXT2-positive cases and 31 (32.3%) of 96 EXT1/EXT2-negative cases were class 5 LMN with proliferative features (class 3 or 4) (Table 6). At the time of diagnosis of LMN, there was no statistically significant difference between the two groups, regarding SCr, hematuria, proteinuria, or presence of proteinuria ≥3.5 g/d. However, in EXT1/EXT2-negative patients, kidney biopsy specimens showed a significantly higher median of global glomerulosclerosis and IFTA (P=0.01 and P=0.003, respectively). At the end of the follow-up, there were no differences between groups on the median SCr or proteinuria ≥3.5 g/d. EXT1/EXT2-positive patients with LN did not evolve to ESKD during the course of the disease (0.0% versus 22.6%; P=0.04), whereas EXT1/EXT2-negative patients developed ESKD in a mean time of 93 months (P=0.03) (Figure 4C). Using multivariable Cox regression, we determined that the predictive factors for the development of ESKD at 10 years were male sex (HR, 8.35; 95% CI, 1.11 to 62.6; P=0.04) and SCr level at the time of kidney biopsy (HR, 1.53; 95% CI, 1.04 to 2.25; P=0.03) (Table 7).
Discussion
Exostosins (EXT1/EXT2) are glycosyltransferases that exist as a heterodimeric copolymerase complex responsible for synthesis of the heparan sulfate chain in the GBM.11 Recently, EXT1/EXT2 were identified as novel, biomarker/putative antigens present in approximately 10%–15% of PLA2R-negative MN. The vast majority (>80%) of EXT1/EXT2-positive MN were associated with autoimmune findings on the basis of kidney biopsy or laboratory findings, or a known underlying autoimmune disease.9 However, only nine patients with SLE with EXT1/EXT2-positive LMN were included in the study. The aim of this study was to determine the prevalence, clinicopathologic findings, and outcomes of EXT1/EXT2-positive and EXT1/EXT2-negative MN in a large series of patients with LMN patients.
In this study of 374 patients with LMN, we confirm that a subset (32.6%) were positive for EXT1/EXT2. Our study shows that patients with EXT1/EXT2-positive LMN have less chronicity on kidney biopsy and lower SCr, but are more likely to have proteinuria ≥3.5 g/d at presentation, and on follow-up, develop a markedly decreased incidence of ESKD compared to patients with EXT1/EXT2-negative LMN. The difference in chronicity between the EXT1/EXT2-positive and EXT1/EXT2-negative LMN was noted in all groups, i.e., overall LMN with or without class 3/4 LN, pure LMN, and LMN with class 3/4 LN. Similarly, ESKD developed more frequently in EXT1/EXT2-negative LMN in all groups, i.e., overall LMN with or without class 3/4 LN, pure LMN, and LMN with class 3/4 LN, compared with EXT1/EXT2-positive LMN.
These findings have significant bearing when considering the high burden of SLE in worldwide, and in particular North America, where the prevalence of SLE is 241/100,000.12 Furthermore, the mortality rate is increased to approximately 2.6-fold in SLE, and the risk of mortality is the highest in patients with kidney disease.13 LN is the most common renal manifestation in SLE: almost 10% of the patients will develop ESKD, and 20% of LN biopsies show LMN with or without concurrent class 3/4 LN.14–16 Our findings show that EXT1/EXT2-positive LMN is a subgroup in LMN that is less likely to develop ESKD, and identification of this subgroup is crucial to the management and prognosis of LMN.
The obvious question that arises is why do patients that are EXT1/EXT2-positive have better outcomes, despite higher proteinuria at presentation? How do EXT1/EXT2 protect against disease progression in LMN? Exostosins are present in the podocyte Golgi apparatus, where they are responsible for the glycosylation of heparan sulfates that are eventually transported to the GBM.17,18 The exostosins have a short N-terminal cytoplasmic tail, a single transmembrane domain, a stem/stalk region, and a long globular catalytic C-terminal domain.19,20 Exostosins, like other glycosyltransferases, are secreted into the extracellular medium, including the GBM, in a truncated form.21 We hypothesize that in patients with EXT1/EXT2-positive LMN, the increased secretion of catalytic domain EXT1/EXT2 into the GBM results in increased synthesis of the heparan sulfates, which, in turn, may offer protection from damaging events, such as leukocyte infiltration, complement activation, cytokine production, etc. (Figure 5). EXT1/EXT2, along with heparan sulfates, may coat the immune complexes and restrict the immune deposits from generating an inflammatory response. Indeed, loss of heparan sulfates in GBM has been associated with development of proteinuria,22 and dysregulation of the complement pathways by impairing complement regulation by complement factor H.23 Furthermore, it was recently shown that heparan sulfates can act as clearance receptors for aberrant extracellular proteins, thus facilitating removing of the immune complexes and proteins involved in the immune response.24 It is likely that EXT1/EXT2 is secreted by the podocytes because EXT1/EXT2 is present in only LMN in the setting of subepithelial deposits, and is not secreted by mesangial cells or endothelial cells because EXT1/EXT2 is absent in class 1–4 LN, where the deposits are mesangial or subendothelial in location. Studies are required to confirm this hypothesis and the events leading to overproduction of EXT1/EXT2 in this subset of patients with LMN. Furthermore, the difference in accumulation of heparan sulfates in the GBM between these two groups needs to be evaluated.
Figure 5.
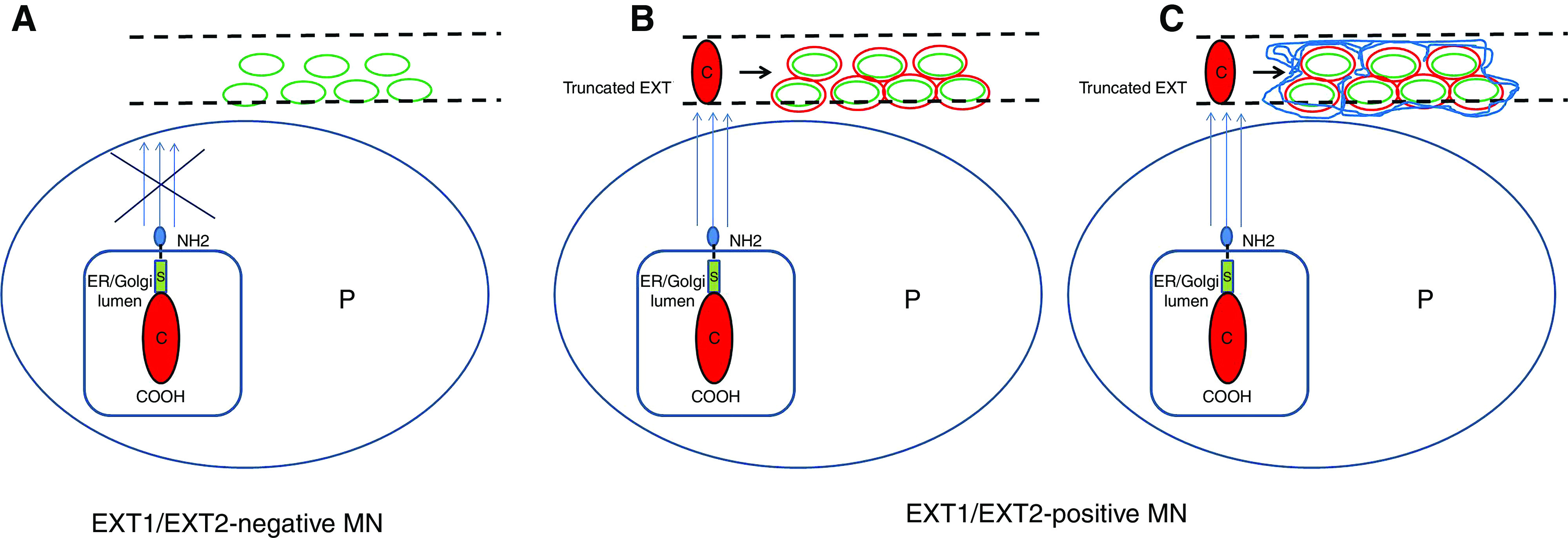
Schematic of EXT1/EXT2-positive and EXT1/EXT2-negative LMN. (A) EXT1/EXT2-negative LMN. There is no increased secretion of EXT1/EXT2 into the glomerular basement membrane (GBM). (B and C) EXT1/EXT2-positive LMN. (B) In EXT1/EXT2-positive LMN, there is increased secretion of the catalytic domain of exostosin into the GBM that is part of the immune complexes. (C) EXT1/EXT2 generate more heparan sulfates in the GBM and those coating the immune complexes. Red circle indicates exostosin, blue lines indicate increased expression of heparan sulfates in the GBM and around the deposits, green indicates immune complexes, and dashed lines indicate GBMs. C, catalytic domain of exostosin; P, podocyte; S, stalk.
Our findings suggest that all cases of LN and LMN should be stained for EXT1/EXT2. The EXT1/EXT2-positive cases in LN may help detect an unknown component of LMN. In addition, it is reasonable to just stain for EXT1 instead of staining for both EXT1 and EXT2, because no single case showed isolated positivity, and EXT1 appears brighter than EXT2. Finally, most cases of LMN were not rebiopsied. In our cohort, only four LMN cases were rebiopsied, of which three were EXT1/EXT2-positive and one was EXT1/EXT2-negative. None of the EXT1/EXT2-positive patients with LMN showed progression of the chronicity indices, whereas the single EXT1/EXT2-negative patient with LMN showed progression of chronicity indices. However, the rebiopsy data needs to be interpreted with caution because the number of rebiopsy cases is very limited.
A limitation of this study is that this is a retrospective study of LMN biopsy specimens, and detailed follow-up was available in 160 (42.8%) of the 374 patients with LMN. Also, specific treatment details along with length of treatment were not available in many patients.
To summarize, 32.6% of LMN patients were positive for EXT1/EXT2. The extent of chronic changes, including global glomerulosclerosis and tubular atrophy and interstitial fibrosis, in EXT1/EXT2-positive LMN are less compared with EXT1/EXT2-negative LMN. EXT1/EXT2-positive LMN rarely progress into ESKD, compared with EXT1/EXT2-negative LMN.
Disclosures
L. Cornell reports being a scientific advisor or member with Minnesota Medical Association; and other interests/relationships with Elsevier. F. Fervenza reports consultancy agreements with Alexion Pharmaceuticals, Alnylam, and ByoCrystal; research funding from Chemocentryx, Genentech, Janssen Pharmaceutical, and Questcor/Mallinckrodt; honoraria from UpToDate; being a scientific advisor or member with Kidney International, JASN, Nephrology Dialysis Transplantation, and UpToDate. J. Grande reports being a scientific advisor or member of the Editorial Board for Biochemistry and Molecular Biology Education. S. Sethi reports honoraria from teaching, grand rounds, and lectures; honoraria from UpToDate; and reviewing slides for a study for Novartis. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The authors thank the Mayo Clinic Genome Facility-Proteomics Core, a shared resource of the Mayo Clinic Cancer Center (NCI P30 CA15083), Department of Laboratory Medicine and Pathology and the Pathology Research Core, Mayo Clinic. The authors also thank Ms. Katelyn A. Reed at the Research and Innovation Office, Department of Laboratory Medicine and Pathology, Mayo Clinic, for her help in acquiring the slides and reports for this study. The study was partly presented at the American Society of Nephrology Annual Meeting, Washington D.C., November 2019 (abstract FR-OR093).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Glomerular Exostosin Deposits in Membranous Lupus Nephritis—a Dialogue,” on pages 525–526.
References
- 1.Couser WG: Primary membranous nephropathy. Clin J Am Soc Nephrol 12: 983–997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makker SP, Tramontano A: Idiopathic membranous nephropathy: an autoimmune disease. Semin Nephrol 31: 333–340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco P, Debiec H: Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet 385: 1983–1992, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al.: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al.: Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 97: 163–174, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A, et al.: Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int 98: 1253–1264, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Anders H-J: Nephropathic autoantigens in the spectrum of lupus nephritis. Nat Rev Nephrol 15: 595–596, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al.: Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 30: 1123–1136, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al.: Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 93: 789–796, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Rops AL, Loeven MA, van Gemst JJ, Eversen I, Van Wijk XM, Dijkman HB, et al.: Modulation of heparan sulfate in the glomerular endothelial glycocalyx decreases leukocyte influx during experimental glomerulonephritis. Kidney Int 86: 932–942, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Stojan G, Petri M: Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol 30: 144–150, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YH, Choi SJ, Ji JD, Song GG: Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus 25: 727–734, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Almaani S, Meara A, Rovin BH: Update on lupus nephritis. Clin J Am Soc Nephrol 12: 825–835, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alarcón GS: Multiethnic lupus cohorts: what have they taught us? Reumatol Clin 7: 3–6, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Huong DL, Papo T, Beaufils H, Wechsler B, Blétry O, Baumelou A, et al.: Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine (Baltimore) 78: 148–166, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Busse-Wicher M, Wicher KB, Kusche-Gullberg M: The exostosin family: proteins with many functions. Matrix Biol 35: 25–33, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Ahn J, Lüdecke HJ, Lindow S, Horton WA, Lee B, Wagner MJ, et al.: Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nat Genet 11: 137–143, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Duncan G, McCormick C, Tufaro F: The link between heparan sulfate and hereditary bone disease: finding a function for the EXT family of putative tumor suppressor proteins. J Clin Invest 108: 511–516, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick C, Duncan G, Goutsos KT, Tufaro F: The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci U S A 97: 668–673, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulson JC, Colley KJ: Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem 264: 17615–17618, 1989 [PubMed] [Google Scholar]
- 22.van den Born J, van den Heuvel LPWJ, Bakker MAH, Veerkamp JH, Assmann KJM, Weening JJ, et al.: Distribution of GBM heparan sulfate proteoglycan core protein and side chains in human glomerular diseases. Kidney Int 43: 454–463, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Borza D-B: Glomerular basement membrane heparan sulfate in health and disease: A regulator of local complement activation. Matrix Biol 57–58: 299–310, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itakura E, Chiba M, Murata T, Matsuura A: Heparan sulfate is a clearance receptor for aberrant extracellular proteins. J Cell Biol 219, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


