Figure 2.
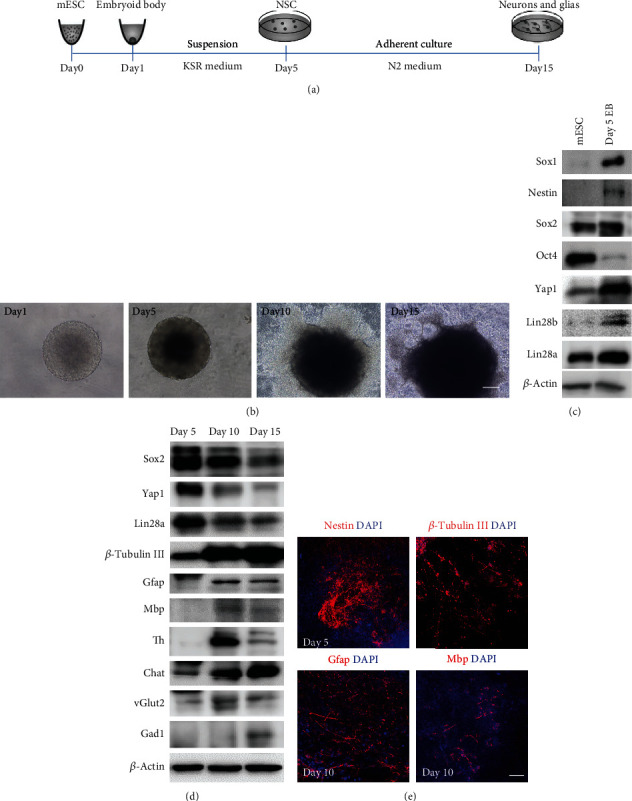
Establishment of the in vitro differentiation protocol from mouse ESCs to neuronal and glial lineage cells. (a) A schematic drawing of the direct differentiation assay from mouse ESCs to neuronal and glial lineage cells. ESCs were cultured under feeder-free condition with 2i + LIF medium for 1 day and then disassociated to single cells and quickly aggregated in differentiation medium for one day. After 5 days of suspension culture in KSR medium, the aggregates were subjected to adhesion culture for another 10 or 15 days in N2 medium. (b) Phase-contrast microscopy of mouse ESC differentiated cells on days 1, 5, 10, and 15. Scale bar, 200 μm. (c) Western blot analyses of total proteins from mESC differentiated cells on day 0 and 5 using the indicated antibodies. Mouse ESC pluripotent markers: Oct4 and Sox2, neural stem cell markers: Sox1, Nestin, and Sox2. (d) Western blot analyses of total proteins from mouse ESC differentiated cells on days 5, 10, and 15 using the indicated antibodies. Neuronal marker: β-tubulin III, glial markers: Gfap and Mbp, neuronal subtype markers: Th, vGlut2, Gad1, and Chat. (e) Immunofluorescence staining of the neural stem cell marker Nestin on day 5, neuronal marker β-tubulin III and glial markers Gfap and Mbp on day 10. Cell nuclear was stained with DAPI. Scale bar, 200 μm.
