Abstract
Paraneoplastic neurologic diseases (PND) are rare but can occur in patients with common malignancies including breast cancer. In patients with hormone receptor (HR)-negative human epidermal growth factor receptor 2 (HER2)-positive breast cancer, PND have been reported in association with anti-Yo antibodies and with clinical presentation of paraneoplastic cerebellar degeneration. We describe the case of a woman with progressively altered mental status and seizures, ultimately requiring admission. Based on her clinical presentation, imaging findings, and evidence of neural-directed antibodies in her serum and cerebrospinal fluid, she was diagnosed with paraneoplastic limbic encephalitis (LE) due to an underlying HR-negative, HER2-positive breast cancer. She showed a transient response to immunosuppression but had more significant improvement after surgical resection and initiation of chemotherapy along with HER2-directed therapy. To the best of our knowledge, this is the first documented case of paraneoplastic LE in a patient with HR-negative, HER2-positive breast cancer likely caused by the production of an unclassified anti-neuronal antibody.
Keywords: Autoimmunity; Breast neoplasms; ERBB2 protein, human; Limbic encephalitis; Paraneoplastic syndromes; Nervous system
INTRODUCTION
Paraneoplastic neurologic diseases (PND) including limbic encephalitis (LE) have been reported to occur in association with breast cancer for many decades, although its incidence is quite rare [1]. Breast cancer-related neurologic paraneoplastic syndromes can have diverse presentations, including sensory and motor-type neuropathies, paraneoplastic cerebellar degeneration (PCD), opsoclonus-myoclonus syndrome, stiff person syndrome, encephalomyelitis, and paraneoplastic retinopathy [1]. Because breast cancer-related paraneoplastic syndromes are uncommon, only limited data are available to support their association with specific breast cancer subsets, presence of certain histological subtypes, advanced clinical stage, or lymphovascular invasion [1].
The diagnosis of a paraneoplastic neurological disorder requires the presence of neurological symptoms, diagnosis of cancer within 4 years, exclusion of other neurological disorders, and at least one of the following findings: cerebrospinal fluid (CSF) analysis showing inflammation with negative cytology, brain magnetic resonance imaging (MRI) demonstrating characteristic hyperintensity signals on T2-weighted fluid attenuated inversion recovery images of the medial temporal lobe(s), or epileptic activity in the temporal lobes by electroencephalogram (EEG) [1,2]. The presence of autoantibodies in the blood or CSF is not necessary for diagnosis. Interestingly, neuron-specific autoantibodies are only found in approximately 60%–70% of patients with clinically diagnosed paraneoplastic syndromes associated with breast cancer [1]. Therefore, antibody testing may be helpful if positive, but the absence of antibodies cannot rule out paraneoplastic neurologic syndrome [1].
Herein, we report a unique case of a patient who presented with rapid-onset altered mental status and temporal lobe seizures along with no other obvious neurologic disorder based on extensive imaging as well as CSF and serum analyses in the setting of a new diagnosis of locally advanced hormone receptor (HR)-negative, human epidermal growth factor receptor 2 (HER2)-positive breast cancer.
CASE REPORT
A 56-year-old woman was admitted to the hospital for psychosis, paranoia, hallucinations, and seizures. Two months before admission, she presented to her primary care physician with right upper extremity numbness and an MRI of the cervical spine that revealed degenerative disc disease. During the following weeks, she developed paranoia, psychosis, and hallucinations, leading to admission at a psychiatric facility. According to the evaluation and treatment of her psychiatric symptoms, the patient was transferred to a medical facility for further workup and to rule out organic causes.
Her past medical history included insomnia treated with zolpidem, but no prior psychiatric disorder. Surgical history was notable for left knee arthroscopy. Her family history included Alzheimer's dementia in her father and a brain tumor in her mother. The patient was living independently and was working before admission. She was a former smoker who did not consume alcohol, marijuana, or other drugs.
On initial presentation to the medical facility, EEG demonstrated focal subclinical seizures originating from the left temporal region. Continuous EEG confirmed frequent seizures, and she was started on multiple anti-epileptic drugs (AEDs), including fosphenytoin, phenytoin, lacosamide, divalproex, and levetiracetam. Her seizures were refractory to medical therapy, and the patient required intubation for airway protection. Brain MRI demonstrated no acute intracranial findings, including no findings specific for encephalitis but incidentally revealed a small right superior frontoparietal paramedian meningioma. Infectious workup, toxicology, blood counts, and electrolytes were all within normal limits, and we could not identify a cause for her seizures and changes in mental status. Computed tomography (CT) of the chest, abdomen, and pelvis revealed a 2.5-cm mass in the right breast with associated right axillary lymphadenopathy and no evidence of distant metastatic disease. Diagnostic lumbar puncture was performed and CSF analysis revealed a cell count of 4 white blood cells (WBC) (reference range, < 5 WBC), 324 red blood cells, glucose 59 mg/dL (reference range, < 300 mg/dL), protein 49 mg/dL (reference range, 18–58 mg/dL), oligoclonal bands, and elevated immunoglobulin G (IgG) index. A second CSF sample was obtained several days later and showed similar findings, and viral, bacterial, and fungal cultures were negative. Cytology was negative for malignant cells in both samples. A comprehensive serum paraneoplastic autoantibody panel and a CSF autoimmune epilepsy panel were both negative for autoantibodies (Table 1). However, the staining pattern was suggestive of the presence of an unknown neuron-specific autoantibody.
Table 1. Comprehensive paraneoplastic autoantibody and autoimmune epilepsy work-up.
| Serum paraneoplastic autoantibodies | CSF autoimmune epilepsy labs | ||
|---|---|---|---|
| Test | Result (reference value) | Test | Result (reference value) |
| ANNA-1 (Hu) | Negative (< 1:240 titer) | NMDA-R AB CBA | Negative (negative) |
| ANNA-2 (Ri) | Negative (< 1:240 titer) | VGKC Complex AB IPA | 0 (≤ 0.02 nmol/L) |
| ANNA-3 | Negative (< 1:240 titer) | LGI1-IgG CBA | Negative (negative) |
| AGNA-1 | Negative (< 1:240 titer) | CASPR2-IgG CBA | Negative (negative) |
| PCA-1 (Yo) | Negative (< 1:240 titer) | GAD65 AB Assay | 0 (≤ 0.02 nmol/L) |
| PCA-2 | Negative (< 1:240 titer) | GABA-B-R AB CBA | Negative (negative) |
| PCA-Tr | Negative (< 1:240 titer) | AMPA-R AB CBA | Negative (negative) |
| Amphiphysin AB | Negative (< 1:240 titer) | ANNA-1 | Negative (< 1:2 titer) |
| CRMP-5-IgG | Negative (< 1:240 titer) | ANNA-2 | Negative (< 1:2 titer) |
| P/Q CAL CHAN AB | 0.01 (≤ 0.02 nmol/L) | ANNA-3 | Negative (< 1:2 titer) |
| N-CAL CHAN AB | 0 (≤ 0.03 nmol/L) | AGNA-1 | Negative (< 1:2 titer) |
| ACHR GANG NEUR AB | 0 (≤ 0.02 nmol/L) | PCA-2 | Negative (< 1:2 titer) |
| NEUR K CHAN AB | 0 (≤ 0.02 nmol/L) | PCA-Tr | Negative (< 1:2 titer) |
| STRIATIONAL AB | Negative (< 1:240 titer) | Amphiphysin AB | Negative (< 1:2 titer) |
| CRMP-5-IgG | Negative (< 1:2 titer) | ||
CSF = cerebrospinal fluid; ANNA = anti-neuronal nuclear antibody; AGNA = anti-glial nuclear antibody; PCA = Purkinje cell cytoplasmic antibody; AB = antibody; CRMP-5 = collapsin response-mediator protein-5; IgG = immunoglobulin G; P/Q CAL CHAN = P/Q-type calcium channel; N-CAL CHAN = N-type calcium channel; ACHR GANG NEUR = acetylcholine receptor ganglionic neuronal; NEUR K = neuronal potassium channel; NMDA-R = N-methyl-d-aspartate receptor; CBA = collagen-binding activity; VGKC Complex AB IPA = neuronal voltage-gated potassium channel antibody; LGI1 = leucine-rich gliomainactivated 1; CASPR2 = contactin-associated protein-like 2; GAD65= glutamic acid decarboxylase; GABA-B-R = gamma aminobutyric acid receptor type B; AMPA-R = AMPA receptor.
The patient underwent biopsy of the right breast mass, which revealed invasive ductal carcinoma, grade 3, Ki-67 90%, estrogen receptor and progesterone receptor negative by immunohistochemical staining (IHC), HER2 equivocal with focal 2+ positivity by IHC, and positive with a HER2/CEN17 signal ratio of 3.2 by fluorescent in situ hybridization. The patient was diagnosed with American Joint Committee on Cancer anatomic stage IIB (cT2N1M0) HR-negative, HER2-positive breast cancer.
Given that paraneoplastic encephalitis is concerning in the setting of a new cancer diagnosis, she received empiric steroids and intravenous immunoglobulin (IVIG) with improvement in her seizures, allowing extubation. Her AEDs were weaned to lacosamide 200 mg twice daily and levetiracetam 1,500 mg twice daily, and she was later discharged from the hospital with neurology and oncology follow-up to receive breast cancer treatment.
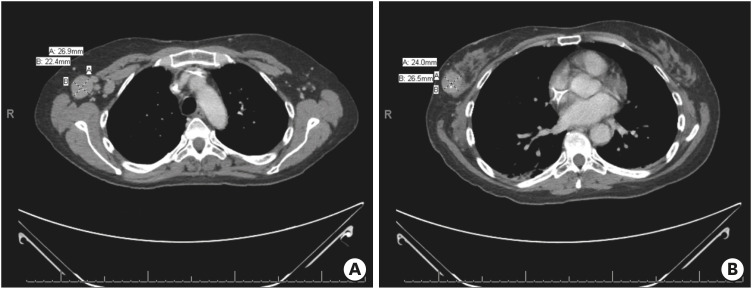
Unfortunately, the patient experienced increased seizures shortly after hospital discharge and was admitted to the neurology service at our tertiary referral center with presumed paraneoplastic encephalitis. Plasma-exchange was initiated, and oncology was consulted for assistance in inpatient management. Physical examination revealed that the woman had anxiety and appeared her stated age of 56; a firm, non-tender, 3-cm mobile mass was palpated in her superior right breast with no overlying skin changes; a 3-cm firm right axillary lymph node was palpated. Neurologically, the patient was oriented appropriately and did not have any focal sensory or motor deficits. She had impaired short-term memory, word-finding difficulty, intermittent non-sensical speech, and paranoia. Repeat brain MRI demonstrated edema and abnormal enhancement involving the left mesial temporal lobe, predominantly in the amygdala (Figure 1). This finding was suggestive of paraneoplastic LE in the clinical context. A CT scan of the chest, abdomen, and pelvis again demonstrated a known right breast cancer with regional axillary lymphadenopathy without evidence of distant metastatic disease (Figure 2). Given these findings, she was diagnosed with LE, a PND, most likely related to her newly diagnosed stage IIB HR-negative HER2-positive breast cancer.
Figure 1. Brain magnetic resonance imaging with and without a contrast agent upon admission to our tertiary care facility. The image demonstrates edema and abnormal enhancement involving the left mesial temporal lobe, predominantly the amygdala, suggestive of paraneoplastic encephalitis given the clinical context.
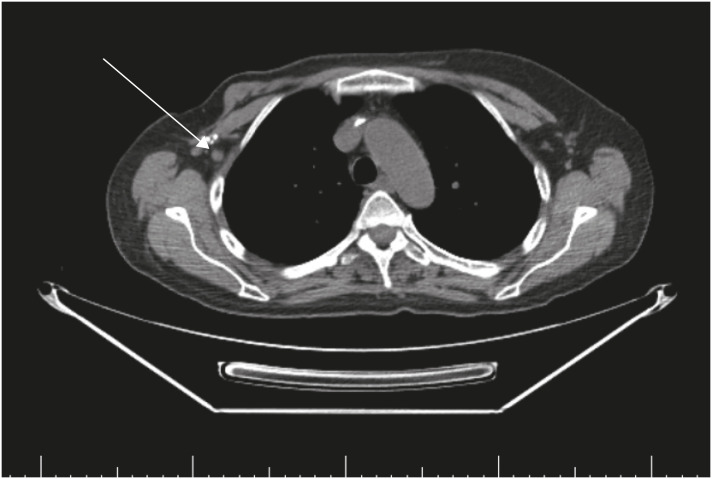
Figure 2. CT of the chest, abdomen, and pelvis. (A) Chest CT image showing right axillary lymphadenopathy and (B) tumor in the lateral right breast with central calcification.
CT = computed tomography.
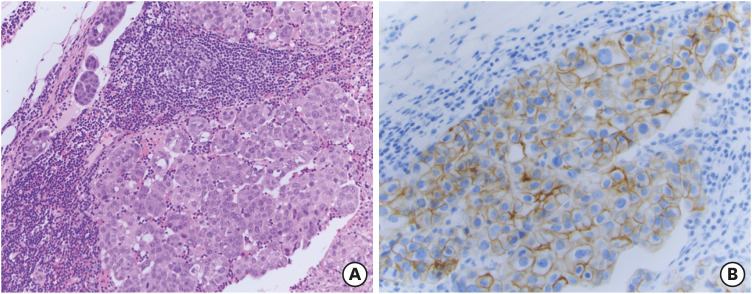
Although non-metastatic breast cancer is almost always treated in the outpatient setting, the patient required inpatient treatment because her breast cancer was felt to be the most likely underlying cause of her LE, and her ongoing psychosis and seizures made discharge unsafe. Following discussion in our multidisciplinary breast cancer tumor board, the patient received one dose of trastuzumab and pertuzumab (HP) followed by right mastectomy and axillary lymph node dissection. The final pathology revealed a 2.7-cm invasive micropapillary carcinoma, grade 3, HR-negative HER2-positive (3+ by IHC) in the right breast, and 6 of 7 positive axillary lymph nodes correlated with pathologic AJCC anatomic Stage IIIA (pT2N2M0) (Figure 3). Neuroendocrine markers were not assessed on the pathological specimen, and the specimens were not assessed specifically for the presence of tumor-infiltrating cells. Within a week of her surgery, she experienced enough improvement in her encephalitis, allowing discharge to a memory care facility under 24-hour supervision. She was able to receive adjuvant chemotherapy with docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) for 6 cycles. During this time, she experienced no deterioration of neurological symptoms but rather had consistent improvement in her mental status. By the end of the 6 cycles of TCHP, her quality of life improved and she no longer required 24-hour supervision. Interestingly, her long-term memory remained relatively intact despite continued difficulty with short-term memory, including remembering day-to-day events and tasks.
Figure 3. Tissue obtained during initial lymph node dissection. (A) Invasive ductal carcinoma at 40× magnification on hematoxylin and eosin staining, and (B) invasive ductal carcinoma with human epidermal growth factor receptor 2-staining (brown) at 40× magnification.
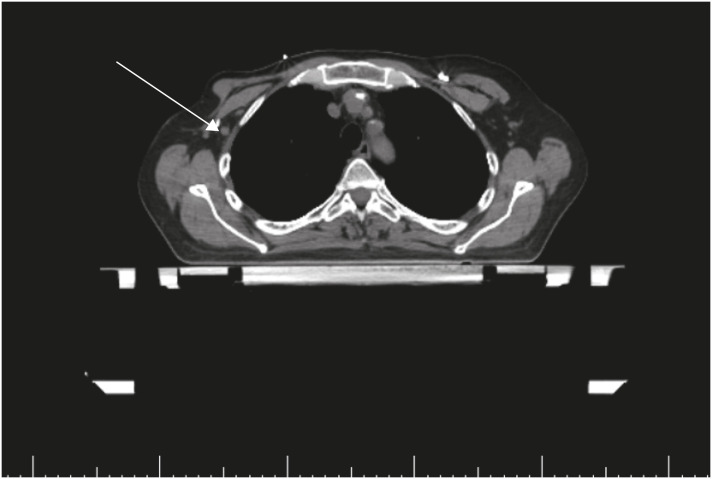
Upon completion of adjuvant chemotherapy, she was referred for radiation oncology considering post-mastectomy chest wall radiation provided to multiple positive lymph nodes. Unfortunately, on CT, she was found to have an enlarged, abnormal axillary lymph node, which, when biopsied, showed metastatic breast cancer that was morphologically consistent with her primary tumor (Figure 4). PET/CT was performed for re-staging and demonstrated right axillary adenopathy, but there was no other evidence of metastatic disease (Figure 5). As a result, she underwent repeat axillary lymph node dissection with 3 of 3 positive lymph nodes with extracapsular extension. This was followed by adjuvant radiation to the chest wall and regional lymph nodes. Given her persistent disease after TCHP, the patient was transitioned to adjuvant ado-trastuzumab emtansine in accordance with the KATHERINE trial [3]. Over the course of this treatment, her mental status slowly improved, brain MRIs normalized (Figure 6), and she remained seizure-free. She currently continues to experience deficits in her short-term memory; however, she is able to manage her day-to-day tasks independently.
Figure 4. Radiation simulation computed tomography scan. Persistent right axillary lymphadenopathy is indicated with an arrow.
Figure 5. Re-staging positron emission tomography-computed tomography. The image shows a rounded right axillary lymph node (9 mm × 8 mm) demonstrating no abnormal uptake and slightly increased size before the examination (7 mm × 6 mm).
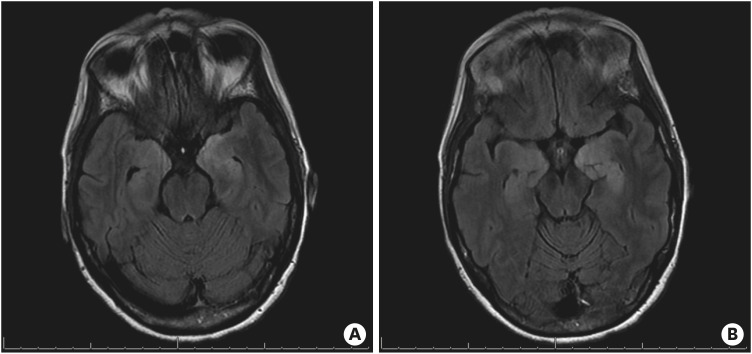
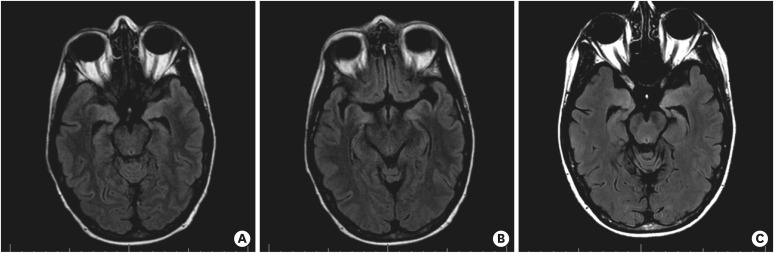
Figure 6. Change in brain MRI findings over time. (A, B) Outpatient follow-up brain MRIs at 5 months from treatment initiation. Since prior, significant improvement in T2 fluid attenuated inversion recovery medial temporal signal abnormality with decreased edema. Minimal signal abnormality involves the bilateral amygdalae. MRI findings suggest improved limbic encephalitis compared to that in two previous MRI studies. (C) Resolved abnormal signal involving the mesial temporal lobes, 9 months from the initiation of treatment.
MRI = magnetic resonance imaging.
DISCUSSION
This patient met all four criteria for the diagnosis of a paraneoplastic neurological disorder, with her mental status changes associated with temporal lobe seizures, a new diagnosis of breast cancer, exclusion of other neurologic disorders with extensive imaging and serum and CSF analyses, and temporal lobe changes on MRI. Her presentation was consistent with LE, and her response to breast cancer treatment also strongly suggests a PND related to newly diagnosed, locally advanced HR-negative, HER2-positive breast cancer.
PNDs are likely initiated as an immune response directed against autoantigens expressed in tumors. Initially, immune cell priming occurs in the tumor microenvironment, as tumor-associated neoepitopes are recognized as foreign by the immune system [1]. The increased cellular proliferation and necrosis that occur in the tumor microenvironment facilitate the expression previously “hidden” neoantigens. Proteinaceous cellular debris may be scavenged by dendritic cells and carried to lymph nodes and lymphoid organs, leading to the amplification of the anti-tumor immune response in B and T cells [1]. In the setting of PND, these exposed tumor-associated neoantigens may be structurally similar to neuronal proteins, leading to an antiauto-neuroantigen response, culminating in autoimmune or paraneoplastic syndromes [1]. In this clinical situation, antineuronal antibodies can often be found in both the serum and the CSF in patients with PND. Additionally, target paraneoplastic antigens are often expressed in both the implicated tumor and the affected parts of the nervous system [1].
Several histological and laboratory findings suggest and support immune-mediated pathology for PND. Autopsy and biopsy specimens obtained from the nervous system in patients with PND typically show neuronal loss in the affected areas associated with infiltration by B cells and CD4+ T-helper cells in the perivascular spaces and infiltration of cytotoxic CD8+ T cells in the interstitial spaces [1]. CSF examination often demonstrates increased nucleated cells, oligoclonal bands, and intrathecal IgG synthesis, consistent with an inflammatory or immune-mediated process [1]. Furthering our understanding of the pathogenesis of PND has been the advent and widespread use of immune checkpoint blockade. One would hypothesize that this class of medication is very active against tumors underlying PND, which are often densely infiltrated with tumor-infiltrating lymphocytes, but they have unfortunately been repeatedly reported to worsen or even initially reveal PND. In support of an immune-mediated process, tumors associated with PND are often small [1]. In some instances, a tumor may not exist at all in the case of spontaneous remissions at the time of neurological presentations, suggesting that some PND without a detectable tumor may actually result from an immune-mediated elimination of the primary tumor [1]. In such circumstances, one could argue that CNS dysfunction actually reflects an effective immune response to systemic cancer [1].
The pathogenic role of paraneoplastic antibodies has been proven for those that are directed against easily accessible antigens located at the cell surface, allowing antineuronal antibodies to interfere with neuronal signaling or synaptic transmission. However, it is more difficult to explain the pathogenic role of antibodies directed against intracellularly located paraneoplastic antigens such as the Yo antigen in the cytoplasm, or the Hu and Ri antigens found in the nucleus. In these instances, the cellular immune response to these antigens through cytotoxic T cells is likely responsible for the neurological damage [1]. Experimental models and analyses of autopsied and biopsied tissues suggest that CD8+ cytotoxic T cells are at the core of PNDs for which the antigens of pathogenic antibodies are within the cell [1]. While intracellular antigens are not readily accessible to an immune incursion, peptides from intracellular proteins are displayed on major histocompatibility complex class I molecules, which are then available to peptide-specific cytotoxic T cells [1]. It stands to reason then that antibodies targeting intracellular antigens, which can be found in the serum and CSF, are not pathogenic, but rather are markers of a T cell-effected neuronal damage process [1].
The most common anti-neuronal antibodies in paraneoplastic LE are anti-Hu (ANNA-1), followed by anti-Ta (PNMA2) and anti-Ma (PNMA1/2) [4]. The most common anti-neuronal autoantibodies seen in patients with breast cancers are anti-Yo (Purkinje cell cytoplasmic antibody type 1), amphiphysin-IgG, and anti-Ri (ANNA-2) [2]. PND associated with HER2-positive breast cancers, which account for approximately 15% of all breast cancer diagnoses, have almost exclusively been reported to have anti-Yo antibodies [5]. Rojas-Marcos et al. [5] published a retrospective review of 27 patients with breast cancer and anti-Yo antibodies, assessing HER2-positivity, which was found in 26 (96.3%) of them, whereas only 2 of 19 (10.5%) patients with anti-Ri-associated PND were found to be HER2-positive. A thorough review of the literature reveals several case reports reporting PNDs in HER2-positive breast cancer, the majority of which are locally advanced, have anti-Yo antibodies, and are classified as PCD (Table 2). We were unable to find any prior reports of HER2-positive breast cancer with associated LE. In reviewing Murphy et al's [2] series of 56 breast cancer patients with PND, the majority of patients had stage II disease (26 patients, 46.4%), but no patients had HR-negative, HER2-positive breast cancer, and only 1 patient had a previously undefined anti-neuronal antibody, which resulted in cerebellar ataxia and peripheral neuropathy. To the best of our knowledge, our patient represents the first documented case of a HER2-positive breast cancer underlying paraneoplastic LE. It also represents a unique case in the presence of an anti-neural antibody associated with this breast cancer subtype that is rare enough that it has not been further classified or accepted into the standard paraneoplastic neurological autoimmune workup.
Table 2. Case reports detailing HER2-positive breast cancer PNDs.
| Reference | HR/HER2 status | Histology | Stage | Antineuronal antibody present | PND syndrome |
|---|---|---|---|---|---|
| Olmez et al. [6] | HR+/HER2+ | IDC | IIA | Anti-Ri | Opsoclonus |
| Myoclonus | |||||
| Le May et al. [7] | HR−/HER2+ | IDC | IIB | Anti-Yo | PCD |
| Dalmau et al. [8] | HR−/HER2+ | IDC | IIA | Anti-Yo | PCD |
| Diamanti et al. [9] | HR+/HER2+ | IDC | IIIC | None | LMND |
| Ogita et al. [10] | HR−/HER2+ | IDC | IIIC | Anti-Yo | PCD |
| Dorn et al. [11] | HR−/HER2+ | IDC | IIIA | Anti-Yo | PCD |
| Rupasinghe and Butler [12] | Unknown HR/HER2+ | Poorly differentiated adenocarcinoma | IIA | Anti-Yo | PCD |
| Frings et al. [13] | HR−/HER2+ | ILC | IIIB | Anti-Yo | PCD |
| Sancho et al. [14] | HR+/HER2+ | IDC | IIB | Anti-Yo | PCD |
HER2 = human epidermal growth factor receptor 2; PND = paraneoplastic neurologic disorder; HR = hormone receptor; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; PCD = paraneoplastic cerebellar degeneration; LMND = lower motor neuron disease.
The most effective therapy for cancer-related PND is the treatment of the underlying malignancy. Gultekin et al. [4] reported 50 patients with PND, including four with breast cancer, showing that treatment of the tumor was more effective in improving neurological outcomes than the use of immunosuppression. Improvement was observed in 38% of anti-Hu patients, 30% of anti-Ta patients, and 64% of patients without these antibodies [4]. In a separate case series, Candler et al. [15] reported that 14% of patients (9/64) with PND had an associated breast cancer (of unknown subtype) and that the only treatment that led to improvement or stabilization of neurologic function was cancer treatment. Aside from cancer therapy, numerous treatment modalities have been investigated in PND and have been found to have limited efficacy. Despite this, immunosuppressive therapy is often used as a bridge to more definitive anticancer therapy. This is especially the case in patients diagnosed with PND as the first symptom of malignancy and before a formal diagnosis. Unfortunately, most patients with PND do not achieve full recovery even with anticancer therapy, and treatment of the underlying malignancy in PND can often stabilize only neurological symptoms, rather than lead to consistent long-term improvements. Finally, the prognosis of PND remains poor, and generally, any level of stabilization or improvement in neurologic symptoms that occurs within the first few months of diagnosis, and treatment becomes a new baseline for these patients.
While our patient initially had slight transient improvements with immunosuppression in the form of IVIG, steroids, and plasma exchange, real improvement was not seen until she underwent surgical resection and began receiving systemic therapy. The decision to proceed with surgical resection upfront as compared to initiating neoadjuvant chemotherapy with TCHP immediately was challenging. Our usual standard of care would be to proceed with neoadjuvant chemotherapy in the setting of node-positive, HER2-positive breast cancer. This approach can often lead to breast-conserving surgery and may even downstage the axilla, allowing for sentinel lymph node biopsy rather than axillary lymph node dissection in select patients. The use of neoadjuvant chemotherapy also allows the use of pathologic complete response as a prognostic marker that strongly correlated with improved disease-free and overall survival and tailoring adjuvant HER2-directed therapy based on response.
In our patient, however, the underlying mechanisms of PND and concern for risks associated with administering multiple cycles of cytotoxic chemotherapy in the inpatient setting, given her altered mental status and psychosis, contributed to the multidisciplinary decision to proceed with upfront surgical resection. We considered the potential for worsening of her PND with cytotoxic chemotherapy that could lead to rapid release of onconeuronal antigens and foreign epitopes resulting from a high volume of tumor cell death and breakdown. Surgical resection of the bulk of the tumor was considered as a potential rapid removal of the bulk of tumor cells, contributing to the formation of further autoimmune neuron-specific autoantibodies. It remains uncertain whether our patient may have responded differently to a more standard-of-care approach to breast cancer with neoadjuvant TCHP followed by surgical resection and radiation.
Our patient had residual disease in her axilla after the completion of 6 cycles of TCHP, requiring repeat surgical resection. Given the persistent disease in the absence of metastatic recurrence, we transitioned adjuvant therapy from HP to ado-trastuzumab emtansine in the spirit of the KATHERINE trial [3]. The KATHERINE trial evaluated patients with HER2-positive T1–T4 and N0–N3 breast cancer who had residual disease at the time of resection following treatment with neoadjuvant HER2-targeted therapy and chemotherapy. In this trial, 12 months of adjuvant ado-trastuzumab resulted in improved invasive disease-free survival compared to treatment with trastuzumab alone (hazard ratio, 0.50; 95% confidence interval, 0.39–0.64; p < 0.001). Although our patient received TCHP in the adjuvant setting, she was found to have residual nodal disease after 6 cycles of chemotherapy, and we felt that she would benefit from the changing maintenance adjuvant therapy to ado-trastuzumab emtansine.
This patient's case represents the first explicitly documented case of paraneoplastic LE in a patient with HER2-positive breast cancer due to an unclassified neural autoantibody. Although breast cancer is the most common cancer diagnosed in women, the presence of associated PNDs is less common than the presence of PNDs associated with other malignancies [6]. The HR-negative, HER2-positive breast cancer subtype is less common than other subtypes, and expression of anti-Yo antibodies in the serum or CSF has previously been reported in patients with associated PCD. Our patient had HR-negative HER2-positive breast cancer, yet did not carry anti-Yo antibodies nor did she have PCD, making her case quite unique. Her presentation and requirement for hospitalization meant that she was treated outside the standard of care, and highlights the need for understanding the pathophysiology of these PNDs; recognition of the need for further research into and delineation of atypical unclassified antibodies given the high incidence of patients without well-characterized antibodies; knowledge of the data regarding efficacy of treatment modalities such as immunosuppressive and anticancer therapies; and the need for tailoring treatment for a specific patient and their specific cancer.
ACKNOWLEDGMENTS
We thank Sharon Sams, MD, for her assistance in providing pathologic images.
Footnotes
Funding: This investigation was supported by the National Institutes of Health (NIH) under Ruth L. Kirschstein National Research Service Award T32CA236734-01. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Shay RC, Diamond JR.
- Resources: Shay RC.
- Supervision: Diamond JR.
- Visualization: Sams SB.
- Writing - original draft: Shay RC.
- Writing - review & editing: Shay RC, Diamond JR, Kagihara JA.
References
- 1.Fanous I, Dillon P. Paraneoplastic neurological complications of breast cancer. Exp Hematol Oncol. 2016;5:29. doi: 10.1186/s40164-016-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy BL, Zalewski NL, Degnim AC, McKeon A, Flanagan EP, Pittock SJ, et al. Breast cancer-related paraneoplastic neurologic disease. Breast Cancer Res Treat. 2018;167:771–778. doi: 10.1007/s10549-017-4566-0. [DOI] [PubMed] [Google Scholar]
- 3.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 4.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 5.Rojas-Marcos I, Picard G, Chinchón D, Gelpi E, Psimaras D, Giometto B, et al. Human epidermal growth factor receptor 2 overexpression in breast cancer of patients with anti-Yo--associated paraneoplastic cerebellar degeneration. Neuro-oncol. 2012;14:506–510. doi: 10.1093/neuonc/nos006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olmez OF, Kinikoglu O, Yilmaz NH, Bilici A, Cubukcu E, Seker M, et al. Anti-Ri-associated paraneoplastic neurological syndrome: initial symptom of breast cancer with HER2 overexpression and treatment by dual HER2 blockade. J Oncol Pharm Pract. 2019;25:1526–1530. doi: 10.1177/1078155218792672. [DOI] [PubMed] [Google Scholar]
- 7.Le May M, Dent S. Anti-Yo antibody-mediated paraneoplastic cerebellar degeneration associated with cognitive affective syndrome in a patient with breast cancer: a case report and literature review. Curr Oncol. 2018;25:e585–e591. doi: 10.3747/co.25.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalmau J, Gonzalez RG, Lerwill MF. Case records of the Massachusetts general hospital. Case 4-2007. A 56-year-old woman with rapidly progressive vertigo and ataxia. N Engl J Med. 2007;356:612–620. doi: 10.1056/NEJMcpc069035. [DOI] [PubMed] [Google Scholar]
- 9.Diamanti L, Quaquarini E, Berzero G, Bini P, Gastaldi M, Franciotta D, et al. Lower motor neuron syndrome in a patient with HER2-positive metastatic breast cancer: a case report and review of the literature. Clin Neurol Neurosurg. 2018;172:141–142. doi: 10.1016/j.clineuro.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Ogita S, Llaguna OH, Feldman SM, Blum R. Paraneoplastic cerebellar degeneration with anti-Yo antibody in a patient with HER2/neu overexpressing breast cancer: a case report with a current literature review. Breast J. 2008;14:382–384. doi: 10.1111/j.1524-4741.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 11.Dorn C, Knobloch C, Kupka M, Morakkabati-Spitz N, Schmolling J. Paraneoplastic neurological syndrome: patient with anti-Yo antibody and breast cancer: a case report. Arch Gynecol Obstet. 2003;269:62–65. doi: 10.1007/s00404-002-0416-2. [DOI] [PubMed] [Google Scholar]
- 12.Rupasinghe J, Butler E. Progressive ataxic gait disorder. J Clin Neurosci. 2007;14:153–157. doi: 10.1016/j.jocn.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Frings M, Antoch G, Knorn P, Freudenberg L, Bier U, Timmann D, et al. Strategies in detection of the primary tumour in anti-Yo associated paraneoplastic cerebellar degeneration. J Neurol. 2005;252:197–201. doi: 10.1007/s00415-005-0635-0. [DOI] [PubMed] [Google Scholar]
- 14.Sancho MI, Lopez MR, Martinez LC, Campelo RG, Facal MS, Budino BS, et al. Subacute cerebellar degeneration as paraneoplastic syndrome: initial symptom of breast cancer with HER2 overexpression. Clin Breast Cancer. 2006;7:79–80. doi: 10.3816/cbc.2006.n.016. [DOI] [PubMed] [Google Scholar]
- 15.Candler PM, Hart PE, Barnett M, Weil R, Rees JH. A follow up study of patients with paraneoplastic neurological disease in the United Kingdom. J Neurol Neurosurg Psychiatry. 2004;75:1411–1415. doi: 10.1136/jnnp.2003.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]