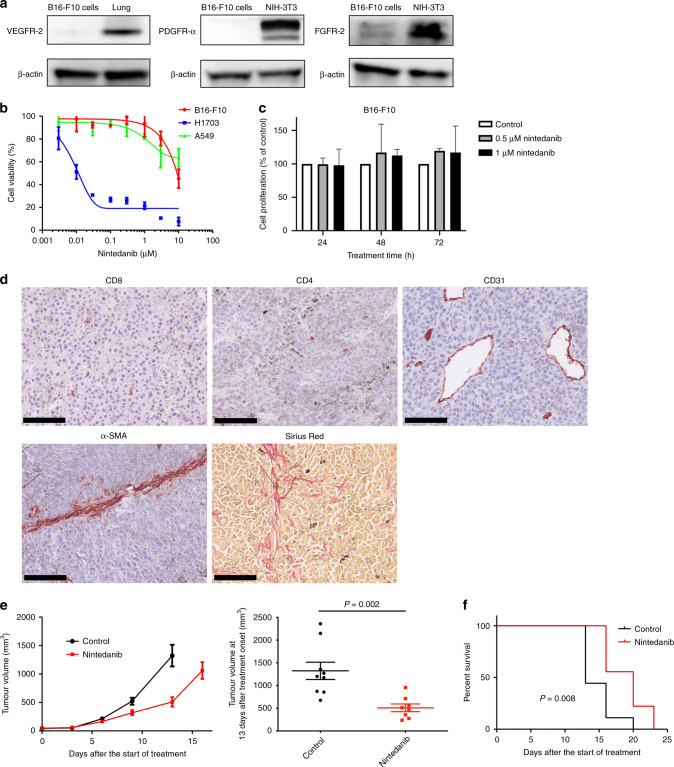
Fig. 1. Nintedanib shows antitumour efficacy without direct cytotoxicity.
a Immunoblot analysis of VEGFR-2, PDGFR-α, FGFR-2 and β-actin (loading control) in B16-F10 cells. Normal mouse lung tissue served as a positive control for VEGFR-2 expression, as did NIH-3T3 cells for PDGFR-α and FGFR-2 expression. b Cell viability assay for nintedanib and either B16-F10, H1703 (positive control) or A549 (negative control) cells. Data are means ± SEM for two independent experiments, each performed with six technical replicates. c Cell proliferation assay for nintedanib and B16-F10 cells. Cells were treated with 0.5 or 1 μM nintedanib for 24, 48 or 72 h, after which living cells were counted by flow cytometry and normalised by those in untreated samples. Data are means + SEM for two independent experiments, each performed with two technical replicates. No significant differences were apparent between untreated and treated samples at the same treatment time (one-way ANOVA with Tukey’s correction for multiple comparisons). d Representative immunohistochemical staining of CD8 and CD4 for T cells, CD31 for microvessels and α-SMA for CAFs as well as representative Sirius red staining of collagen in B16-F10 tumours derived from mice. Scale bars, 100 μm. e Time course of tumour volume (left) as well as tumour volume at 13 days after treatment initiation (right) for subcutaneous B16-F10 tumours treated with nintedanib or vehicle (control). Data are means ± SEM for seven or eight tumours in each group. The P value was determined with the unpaired t-test. f Survival curves for the B16-F10 tumour-bearing mice in e. The P value was determined with the log-rank test.