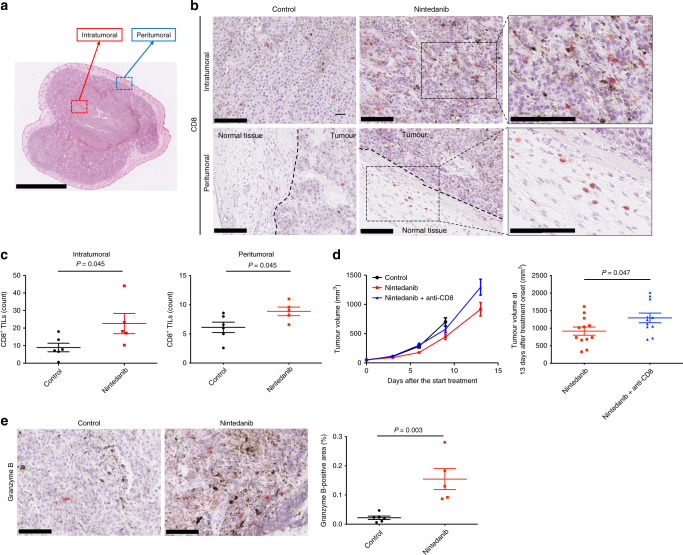
Fig. 3. Nintedanib promotes antitumour immunity in B16-F10 tumours.
a Representative haematoxylin-eosin–stained section of a B16-F10 tumour indicating the intra- and peritumoural borders. Scale bar, 2.5 mm. b Representative immunohistochemical staining for CD8 in intra- and peritumoural regions of B16-F10 tumours derived from mice treated with vehicle or nintedanib for 7 days. Scale bars, 100 μm. c Number of CD8+ TILs in intra- and peritumoural regions of B16-F10 tumours determined from sections as in b. Data are means ± SEM for five or six mice per group. The P values were determined with the unpaired t-test. d Time course of tumour volume (left) as well as tumour volume at 13 days after treatment onset (right) for B16-F10 tumours treated with vehicle or with nintedanib either alone or together with an antibody to CD8 to deplete CD8+ cells. Data are means ± SEM for 11 or 12 tumours in each arm (pooled from two independent experiments). The P value was determined with the unpaired t-test. e Representative immunohistochemical staining (left) as well as the percentage positive area (right) for granzyme B in B16-F10 tumours derived from mice treated with vehicle or nintedanib for 7 days. Scale bars, 100 μm. Quantitative data are means ± SEM for five or six mice per group. The P value was determined with the unpaired t-test.