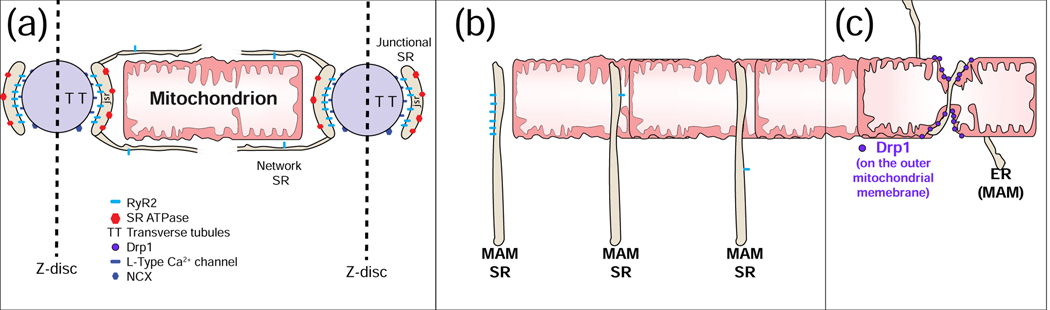
Figure 4. Mitochondrial Associated Membranes (MAMs) and Ca2+ signals.
A. Excitation-contraction coupling (ECC) and MAMs. The sarcoplasmic reticulum (SR) is contiguous with the endoplasmic reticulum (ER) and contribute membranes to MAMs. In cardiac myocytes, [Ca2+]i transient is triggered electrically by action potentials (AP). In ventricular myocytes, there are a very large number of Ca2+ spark sites (~20,000 per cell). Each site includes an array of ~50 SR Ca2+-release channels (ryanodine receptors type 2; RyR2) in the junctional SR that faces a transverse tubule (TT) or the sarcolemma (SL) that become activated upon Ca2+ elevation in the narrow ~15 nm subspace. During cardiac AP, the L-type Ca2+ channels in the TT and SL membranes are depolarization and conduct Ca2+ into the subspace. This high subspace Ca2+ activates the RyR2s enabling Ca2+ release from the SR to produce a Ca2+ spark. B. Three common arrangements of RyR2s in MAMs. Left: A cluster of RyR2s facing away from the mitochondrion, 20 to 100 nm away, similar to those of a Ca2+ spark site. Middle: Isolated RyR2 channel in a MAM facing an OMM at ~15 to 50 nm. These channels are not normally triggered to open. Under quiescent conditions, the opening rate is ~10−4 s−1. Right: Isolated RyR2 in a MAM more than 100 nm away from the mitochondrion. C. Garrote-assisted fission. It has been suggested that mitochondrial fission, normally attributed to the action of Drp1, may be assisted by the encircling action of ER or SR.