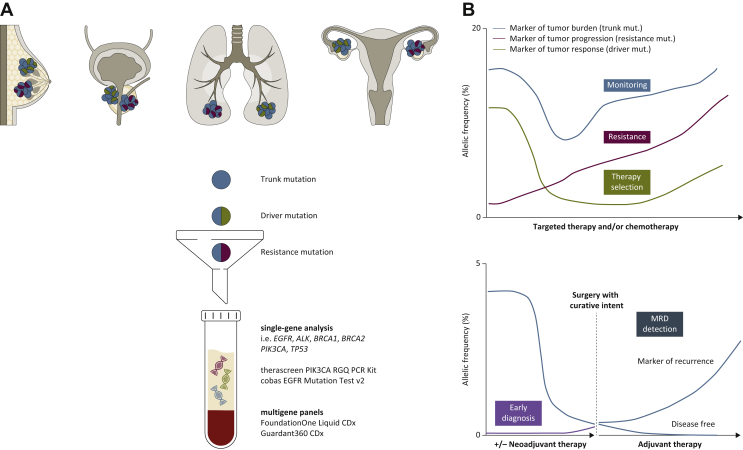
Figure 1.
Plasma cell-free tumor DNA (ctDNA) clinical utility as a liquid biopsy.
(A) ctDNA can be shed by various tumor types, including breast, lung, prostate, and ovarian cancers. Each lesion can harbor different alterations (trunk mutation, blue, shared by all the clones as it occurs early in the tumorigenesis; driver mutation, green, and resistance mutation, red, which can emerge after treatment). Once in the blood, ctDNA can be collected by venipuncture, and the sample is then processed to obtain plasma. After isolation, ctDNA can be assessed to investigate for molecular alterations by two approaches: the single-gene analysis (PCR-based methods such as droplet digital PCR, upper panel), or the multigene panel analysis (next-generation sequencing, lower panel). Four tests have by now been approved by the US Food and Drug Administration (FDA) for clinical use: the therascreen PIK3CA RGQ PCR Kit and the cobas EGFR Mutation Test v2, and FoundationOne Liquid CDx and Guardant360 CDx, for the single-gene and multigene analysis, respectively. (B) ctDNA as a biomarker with different dynamics, where the percentage of mutational allelic frequency is quantified throughout all the collected ctDNA timepoints. Upper panel: ctDNA is assessed for three biomarkers: (i) for patients' monitoring during treatment, with a trunk mutation, as a marker of tumor burden, blue line; (ii) for therapy selection, with a driver mutation, as a marker of tumor response to therapy, green line; and (iii) with a resistance mutation, as a marker of tumor progression and resistance to therapy, red line. Lower panel: ctDNA used as a marker of early tumor detection and diagnosis in patients with early stage cancer or in asymptomatic population (purple line). In addition, ctDNA can be used to detect minimal residual disease (MRD, blue line) in cancer patients undergoing surgery with curative intent, with or without prior neoadjuvant treatment. If ctDNA after surgery is still detected, then the patient could be guided to receive adjuvant therapy and monitored for further disease recurrence; if ctDNA after surgery is not detected, then the patient could be considered disease free.