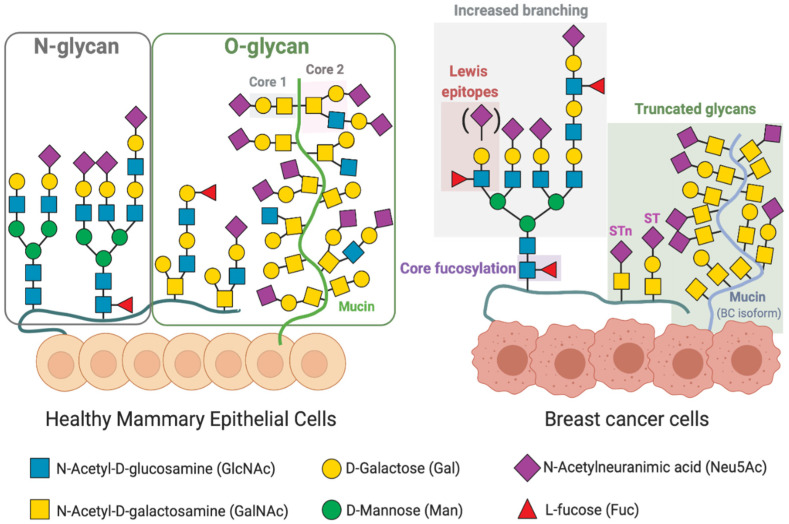
Figure 2.
Altered glycosylation patterns of surface glycoproteins on breast cancer cells. Healthy mammary epithelial cells present at their surface diverse glycans, which are attached by a N-Acetylglucosamine (GlcNAc) to an asparagine (Asn) site—N-glycans—or by a N-Acetylgalactosamine (GalNAc) to a serine/threonine site (Ser/Thr; mucin-type O-glycans). Upon tumorigenesis, BC cells acquire a deregulated synthesis of glycans, which influences interactions between tumour cells and the surrounding microenvironment. Altered glycosylation in BC is characterized by an increase in Lewis antigens (Lewisa and Lewisx; the sialylated Lewisx and Lewisa) and an increase in α1-6-core fucosylation and branching of N-glycans. Additionally, truncated O-glycans are highly expressed in BC usually with terminal sialic acid. Monosaccharides are represented according to the symbol nomenclature for glycans (SNFG) [8,37,39,41].