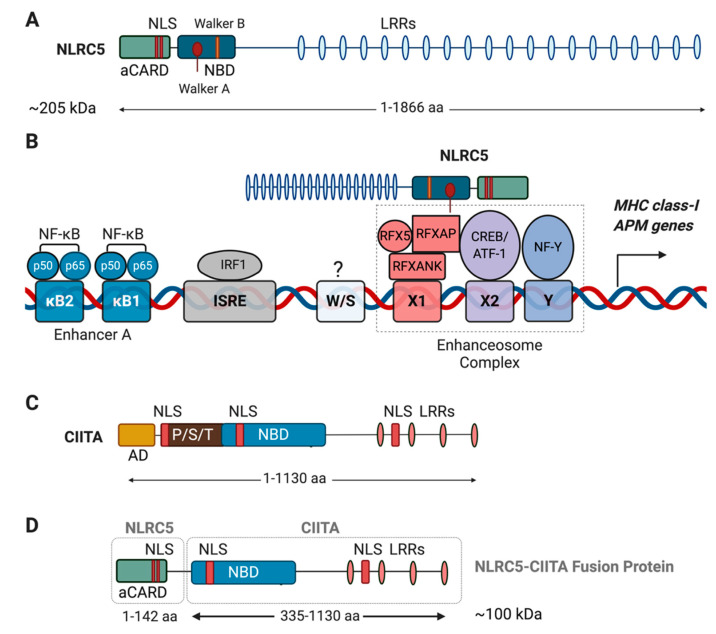
Figure 2.
Structure and transactivation functions of NLRC5. (A) NLRC5 (1866 aa) displays a tri-partite structure consisting of (i) an atypical CARD (aCARD; 1–139 aa) domain with a helix extending to residue 161 and a bipartite Nuclear Localization Sequence (NLS; 121–122, 132–134 aa), (ii) a Nuclear Binding Domain (NBD; 197–369 aa) that harbors Walker A and Walker B motifs (228–235, 303–313 aa) and (iii) twenty Leucine Rich Repeats (LRRs) (589–1866 aa). (B) NLRC5 transactivates MHC-I and APM genes via the SXY module. NLRC5 lacks a DNA binding domain and thus interacts through the enhanceosome complex formed by the transcription factors (RFX proteins, CREB/ATF1, NF-Y) bound to X1, X2 and Y box motifs. RFX5 acts as a key mediator for binding of NLRC5 with the promoter. The LRRs of NLRC5 are compressed for clarity. The MHC-I promoters also harbor an interferon stimulated responsive element (ISRE) and κB consensus sites that bind IRF1 and NF-κB, respectively. (C) Structure of CIITA (NLRA; 1130 aa). CIITA has an acidic activation domain (AD) (1–125 aa), three nuclear localization sequences (141–159, 405–414, 955–959 aa), a P/S/T domain (Pro/Ser/Thr; 126-322 aa), an NBD (336–702 aa) and four LRRs (930–1130 aa). (D) The Kufer laboratory has engineered an NLRC5-CIITA fusion protein containing the aCARD domain of NLRC5 and NBD and LRRs of CIITA. This fusion protein transactivates MHC-I genes as efficiently as the full length NLRC5.