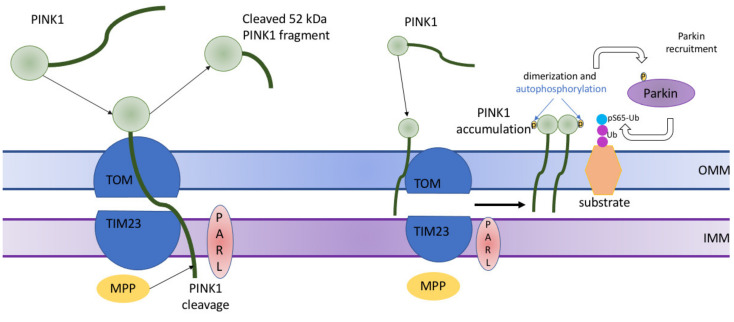
Figure 2.
Brief description of PTEN-induced kinase 1 (PINK1)-mediated stimulation of mitophagy in normal and damaged mitochondria. In normal mitochondria (on the left), PINK1 is imported into mitochondria with the help of translocase of the outer membrane (TOM) complex and translocase of the inner membrane (TIM). At the IMM, PINK1 is initially processed by matrix processing peptidase (MPP), which removes PINK1’s N-terminal mitochondrial targeting signal, and then PINK1 is being proteolytically cleaved by the intermembrane serine protease presenilin-associated rhomboid-like protein (PARL), which cleaves the full-sized PINK1 form of 64 kDa into 60 kDa and 52 kDa fragments. In damaged mitochondria (on the right), PINK1 stores on the outer mitochondrial membrane of only injured mitochondria and is stabilized in a TOM complex (TOM7, TOM40, TOM70, TOM20, and TOM22). PINK1 mediates two various phosphorylations serving for the transformation of the autoinhibited E3-ubiquitin (Ub) ligase Parkin into an active phospho-Ub-dependent enzyme. Then, the direct phosphorylation of Parkin at S65 in the N-terminal Ubl domain occurs, increasing the activity of its E3 ligase. After that, PINK1 triggers the addition of phosphate onto S65 of Ub. This complex regulatory mechanism results in activation of the E3 ligase activity of Parkin, which allows it to ubiquitinate mitochondrial proteins through direct interaction with phospho-Ub conjugates on the mitochondria. OMM—outer mitochondrial membrane; IMM—inner mitochondrial membrane.