Figure F.1.
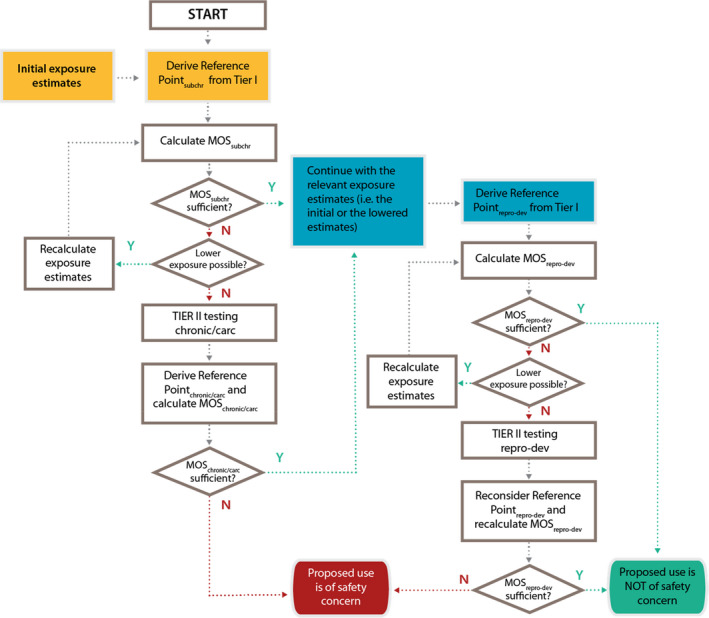
The decision scheme is based on the outcome of the Tier I testing for subchronic repeated dose toxicity and reproductive–developmental toxicity testing in combination with the outcome of the exposure assessment. It is only applicable to applications for the authorisation of new smoke flavouring primary products (for renewal applications see Figure F.2). The scheme is the conceptual representation of the considerations leading to either the identification of needs for Tier II testing or to the conclusion of no concern on the basis of the data available after Tier I. Following Tier II, a final conclusion will be reached, which could be either that there is a safety concern for the smoke flavouring primary product based on the proposed uses and use levels or that there is no safety concern for the primary product based on the proposed uses and use levels.
The decision scheme starts at the top with the derivation of the reference point from the subchronic repeated dose toxicity (subchr) resulting from the Tier I testing (yellow shading). The initial exposure estimate (yellow shading) is also input data that is needed for the calculation of the MOS for subchronic repeated dose toxicity (MOSsubchr) at Tier I. The reference point for reproductive‐developmental toxicity (repro‐dev) after Tier I (blue shading) is consecutive input data which should be combined with the exposure estimate as based on the information initially submitted by the applicant or with a lowered exposure estimate following the Tier I assessment of subchronic toxicity (blue shading). From these the MOS for reproductive/developmental toxicity can be calculated (MOSrepro‐dev). The diamonds in the decision scheme include two types of questions: a) whether the MOSsubchr or MOSrepro‐dev or the MOS for chronic toxicity and carcinogenicity study (MOSchronic/carc) are sufficient to conclude that the primary product can be considered to be of no safety concern under the proposed conditions of use. For more details on the numerical cut‐offs for the MOS, refer to Section 3.3.3; b) whether it is possible to lower the exposure estimates. This could be achieved by refining the exposure estimates (to be done by EFSA during the risk assessment). If this does not result in a sufficiently high MOS, the exposure can subsequently be lowered by lowering the (proposed) use levels and/or by reducing the uses (to be done by the applicant). If the answer to the questions in the diamonds is No (N), i.e. if the MOS are too low and if it is not possible to lower the exposure, a need for additional toxicity testing in Tier II is triggered, either for chronic toxicity and carcinogenicity testing (chronic/carc) and/or for additional testing to follow‐up toxicity effects observed in the EOGRTS (e.g. endocrine‐, neuro‐ and immuno‐toxicity effects). If after the Tier II testing the MOS are still too low, the smoke flavouring primary product is concluded to be of safety concern under the proposed conditions of use. On the other hand, if the answer to the questions in the diamonds addressing the magnitude of the MOS is Yes (Y), it can be concluded that the smoke flavouring primary product is not of safety concern under the proposed conditions of use.
