Figure F.2.
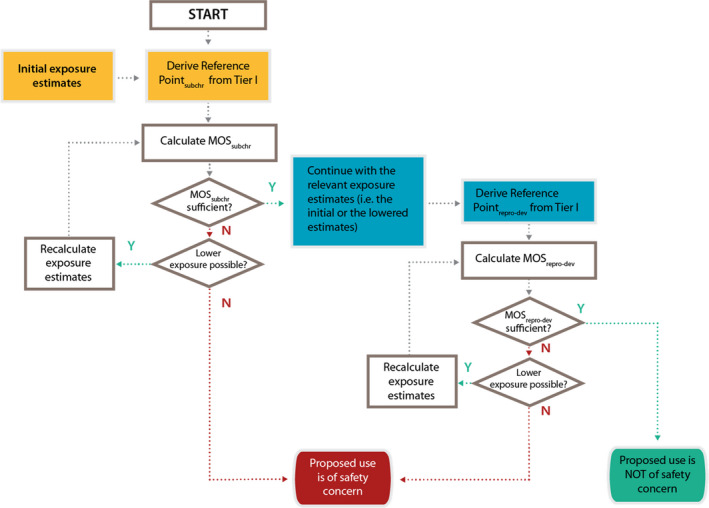
The decision scheme is based on the outcome of the Tier I testing for subchronic repeated dose toxicity and (preferably) reproductive–developmental toxicity testing (EOGRTS) or developmental toxicity (OECD TG 414) in combination with the outcome of the exposure assessment. It is applicable only to renewal applications for smoke flavouring primary products (for applications for the authorisation of new smoke flavouring primary products see Figure F.1). The scheme is the conceptual representation of the considerations leading to a final conclusion, which could be either that there is a safety concern for the smoke flavouring primary product based on the proposed uses and use levels or that there is no safety concern for the primary product based on the proposed uses and use levels. The decision scheme starts at the top with the derivation of the reference point from the subchronic repeated dose toxicity (subchr) (yellow shading). The initial exposure estimate (yellow shading) is also input data that is needed for the calculation of the MOS for subchronic repeated dose toxicity (MOSsubchr). The Reference Point for developmental or reproductive‐developmental toxicity (repro‐dev; blue shading) is consecutive input data which should be combined with the exposure estimate based on the information initially submitted by the applicant or with a lowered exposure estimate following the assessment of subchronic toxicity (blue shading). From these the MOS for reproductive/developmental toxicity can be calculated (MOSrepro‐dev). The diamonds in the decision scheme include two types of questions: (a) whether the MOSsubchr or MOSrepro‐dev are sufficient to conclude that the primary product can be considered to be of no safety concern under the proposed conditions of use. For more details on the numerical cut‐offs for the MOS, refer to Section 3.3.3; (b) whether it is possible to lower the exposure estimates. This could be achieved by refining the exposure estimates (to be done by EFSA during the risk assessment). If this does not result in a sufficiently high MOS, the exposure can subsequently be lowered by lowering the (proposed) use levels and/or by reducing the uses (to be done by the applicant). If the answer to the questions in the diamonds is No (N), i.e. if the MOS are too low and if it is not possible to lower the exposure estimate, the smoke flavouring primary product is concluded to be of safety concern under the proposed conditions of use. On the other hand, if the answer to the questions in the diamonds addressing the magnitude of the MOS is Yes (Y), it can be concluded that the smoke flavouring primary product is not of safety concern under the proposed conditions of use.
