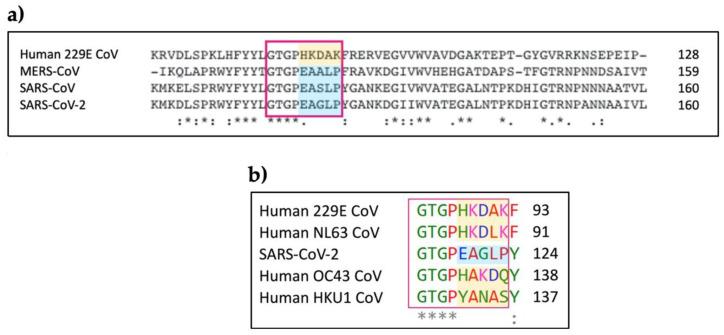
Figure 1.
(a) Sequence alignment of the interacting portion of the C-terminal domain of human coronavirus N proteins. The region interacting with mannose-binding protein-associated serine protease 2 (MASP-2) is highlighted with a red box. While the amino acid composition of this region is highly conserved in highly pathogenic human coronaviruses, significant differences are found in the corresponding region of human coronaviruses associated with mild diseases, exemplified by 229E-CoV (varying portion highlighted in orange). This sequence variation has been proven to abrogate the ability to interact with MASP-2 and induce aberrant complement activation [7]. (b) Sequence alignment between the relevant portion of the C-terminal domain of the SARS-CoV-2 N protein and the corresponding portion of the N proteins of human coronaviruses associated with mild diseases [9]. The region interacting with MASP-2, highlighted with a red box, shows significant differences (varying portion highlighted in orange). N protein sequences were downloaded from UniProt [10]. Sequence alignments were performed with Clustal Omega [11]. In the sequence alignment, ‘*’ indicates conserved residues, ‘:’ indicates conserved substitutions, while ‘.’ indicates semi-conserved substitutions. Red indicates small and hydrophobic residues, blue indicates acidic residues, magenta indicates basic residues, green indicates residues containing hydroxyl or sulfhydryl or amine and G [11].