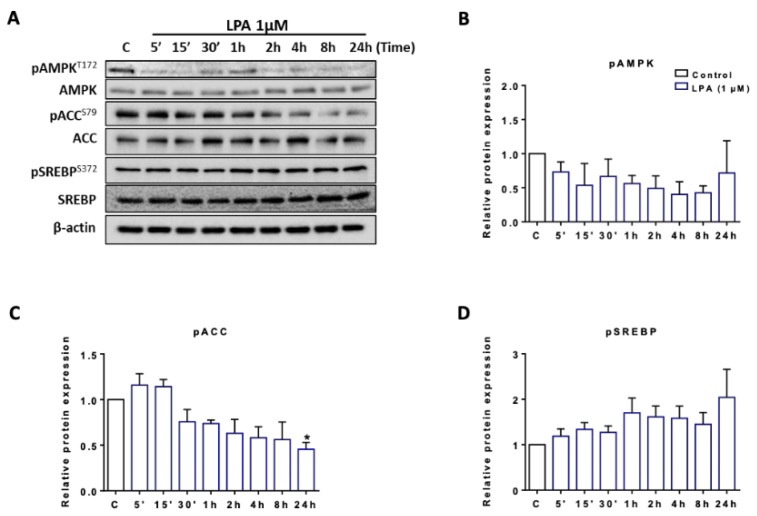
Figure 4.
Phosphorylation status of AMP-activated protein kinase (AMPK), acetyl-CoA carboxylase (ACC), and sterol regulatory element binding protein (SREBP) in LPA treated BV-2 cells. (A) BV-2 cells were serum-starved overnight and incubated in the absence (‘c’) and presence of 1 µM LPA for the indicated times. Phosphorylation states of proteins were detected using Western blot analysis, β-actin served as loading control. One representative blot out of three is shown. Densitometric analyses of immunoreactive bands of (B) phospho AMPK (pAMPK), (C) phospho ACC (pACC), and (D) phospho SREBP (pSREBP) relative to β-actin are shown. Results are presented as mean values ± SEM of 3 independent experiments. (* p < 0.05, compared to control; one-way ANOVA with Bonferroni correction).