Figure 4. LC-MS analysis of in vivo glucosylation of IFNα-Q158N, subsequent in vitro transglycosylation, and enrichment of the transglycosylation product.
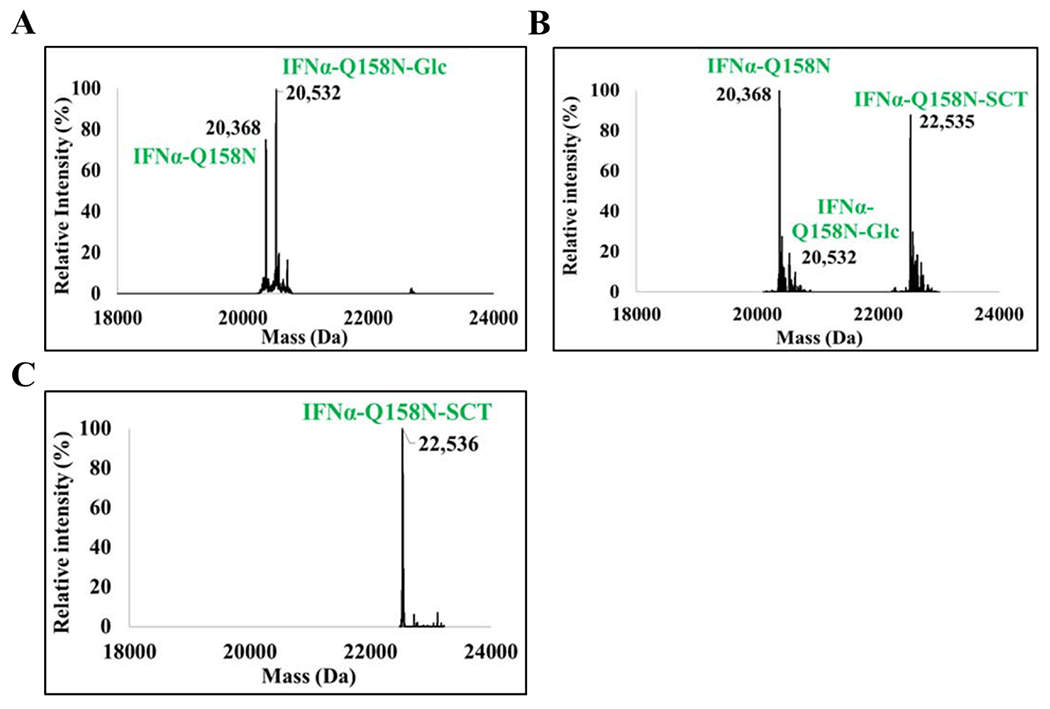
Deconvoluted LC-MS profile of A) IFNα-Q158N co-expressed with ApNGT in E. coli. Calculated mass of IFNα-Q158N with two disulfide bonds, M = 20370 Da. Calculated mass of the glucosylated protein, M = 20532 Da. Additional higher mass peak observed in the profile is an unidentified non-glucose adduct. B) In vitro chemoenzymatic transglycosylation of IFNα-Q158N by EndoCC-N180H. Calculated mass of IFNα-Q158N-SCT, M = 22534 Da. 90% transglycosylation was observed. It should be noted that the protein is only 55% glucosylated resulting in a total yield of 50% sialylated protein. C) The IFNα-Q158N-SCT enriched using Lectenz affinity chromatography. The IFNα-Q158N referred to in this figure is the His-tagged protein.
