Abstract
Background
Scarring central airway stenosis (SCAS) is a potentially life-threatening condition with debilitating symptoms. Interventional bronchoscopy is increasingly used to relieve symptoms in patients with SCAS, but recurrent stenosis is frequently observed. Little data exist on the long-term prognosis of interventional bronchoscopy for SCAS. We aimed to assess the prognostic factors of bronchoscopic interventions in patients with SCAS to optimize treatment.
Methods
This was a retrospective study that enrolled 119 consecutive patients with SCAS from January 2010 to April 2019 at our institution. Long-term clinical success was defined as airway stenosis < 50%, no limitation of physical activity, and a stable condition for > 12 months after the last interventional procedure. We compared patients’ demographics, airway stenosis characteristics, and interventional procedures between the successful and unsuccessful groups, and identified significant predictors of long-term outcome with univariate and multivariate logistic regression.
Results
A total of 119 patients with 577 therapeutic bronchoscopies were included. Seventy-five (63%) patients were considered to have long-term clinical success. Older age, male gender, smoking, elevated C-reactive protein level, subglottic stenosis, stent or T-tube implantation, previous interventional treatment, and multiple procedures per year were potentially associated with unsuccessful long-term outcomes in the univariate analysis. Current smoker status (odds ratio [OR] 5.70, 95% confidence interval [CI] 1.35–24.17, P = 0.018), subglottic stenosis (OR 4.35, 95% CI 1.31–14.46, P = 0.017), and stent implantation (OR 4.96, 95% CI 1.33–18.48, P = 0.017) were associated with decreased odds of long-term success in the multivariate logistic regression analysis. Of note, there was no significant difference in odds of success between former smokers and nonsmokers.
Conclusions
Current smoker status, subglottic stenosis, and stent implantation are independent factors associated with reduced long-term efficacy of interventional bronchoscopy for SCAS. Smoking cessation should be encouraged to improve the outcome of therapeutic bronchoscopy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-021-01434-5.
Keywords: Airway obstruction, Bronchoscopy, Outcome, Subglottic stenosis, Smoking
Background
Scarring central airway stenosis (SCAS) is a life-threatening condition that is predominantly associated with shrinking and scarring in the trachea, left and right main bronchi, and right middle bronchus, which could cause severe dyspnea [1–3]. Many benign etiologies can result in SCAS, such as post-intubation and post-tracheostomy, infectious inflammation, anastomotic stenosis, airway trauma, and foreign body stimulation [4].
Surgical resection and reconstruction used to be the gold standard therapy [5]. However, the surgery is technically challenging, and many patients are either inoperable or unable to tolerate surgery due to their poor condition. Interventional bronchoscopy modalities, including balloon dilation, electrocautery, laser, argon plasma coagulation, cryotherapy, T-tube, and silicone stent implantation, which are less invasive and can rapidly alleviate shortness of breath, have been increasingly used to manage SCAS [6, 7]. A previous study has reported the short-term efficacy of interventional bronchoscopy as up to 98.67% [8]. Nevertheless, SCAS has a high risk of recurrence which occurs in 40–70% of patients [9]. Thus, patients must receive repeated treatments, which significantly influences their quality of life and increases the financial burden on families, rendering it a challenge in interventional pulmonology. Sratakos et al. and Dalar et al. have reported that the long-term efficacy is lower in complex stenosis featuring long stenosis and cartilage involvement [6, 10]. Identifying which subgroups of patients may benefit from the intervention and obtain long-term successful results is paramount. However, little data regarding the long-term prognosis of interventional bronchoscopy for SCAS are available. Therefore, we retrospectively evaluated the long-term clinical outcome in patients who underwent interventional bronchoscopy for SCAS, aiming to assess the prognostic predictors to optimize treatment.
Methods
Patients
We retrospectively reviewed all consecutive patients who underwent interventional bronchoscopy due to SCAS at our institution, a tertiary referral center for interventional bronchoscopy in China, between January 2010 and April 2019. The included patients either had contraindications to surgery or decided to opt for interventional bronchoscopy following a discussion with their surgeons. The inclusion criteria were as follows: (1) diagnosed with SCAS based on bronchoscopy; (2) received interventional bronchoscopic treatment; and (3) followed up for at least 12 months. The study excluded patients with intraluminal malignant tumors, extrinsic compression, dynamic stenosis, active infection, systemic diseases such as relapsing polychondritis and granulomatosis with polyangiitis, and those who were either followed up for less than 12 months or lost to follow-up. The first interventional bronchoscopy treatment was arranged if the patient was symptomatic with more than 50% airway stenosis. Subsequent bronchoscopic therapy was considered for patients with recurrent symptoms, a marked decline in pulmonary ventilatory capacity, or as maintenance treatment determined by the clinicians. Oral informed consent was obtained from all participants and the Institutional Review Board of Peking University First Hospital approved this study (2020209).
Bronchoscopic strategies for the treatment of SCAS
First, an electronic knife or laser was used to cut the scar tissue radially, and rigid bronchoscope or balloon dilation was then conducted to dilate the stenotic airway. Granulation was debrided using electric snare, holmium–yttrium aluminum garnet (Ho:YAG) laser, or cryotherapy. Three 30 to 60-s cycles of cryoablation were given using a freeze–thaw procedure at the granulation site or basilar portion of the scar. After excluding active infection, patients with recurrent stenosis received an intralesional injection of 1 ml diprospan (betamethasone 2 mg/betamethasone dipropionate 5 mg) to prevent restenosis. Silicone stent implantation was performed with recurrent stenosis, and it would be removed if the airway remained stable for at least 1 year or severe stent-related complications appeared. To prevent severe airway laceration, appropriately sized balloons were selected according to the measurement by CT scan. Whenever possible, we avoided treating the stenosis with active infection. Additionally, electrocautery and other thermo-ablations were avoided to minimize further airway injury and granulation hyperplasia.
Outcome
The primary outcome was long-term clinical success at the last visit. Long-term clinical success was defined as airway stenosis < 50%, no limitation of physical activity, and the diameter of the airway is stable for more than 12 months after the last interventional procedure [11]. Long-term clinical success for patients who received stent implantation was also defined according to the criteria above.
Data collection
Clinical information including basic demographics, smoking status, pulmonary comorbidities, symptoms, laboratory examinations at baseline, interventional treatment intervals, the total number of procedures, and non-interventional treatments were extracted from hospital electronic medical records. Data on airway stenosis data, procedure-related data, and complications were extracted from the bronchoscopy report. Structured telephone-based interviews were performed in April 2020. We evaluated the degree of airway stenosis defined by the decrease in cross-sectional area by referring to the Myer–Cotton stenosis grading system [12]. Former smokers and current smokers were distinguished by cigarette cessation before the first interventional bronchoscopy. Carbon dioxide (CO2) retention was defined as an arterial CO2 pressure > 50 mmHg with an arterial oxygen pressure > 60 mmHg on room air. Multiple modalities (such as electrocautery, laser, dilation, cryotherapy, and diprospan injection) could be performed during a single bronchoscopic therapy. The total number of therapies was defined as the number of interventional bronchoscopies regardless of the use of multiple modalities. The cumulative number of each modality was defined as the sum of the use of respective modality in all bronchoscopies regardless of repeated use during one bronchoscopy. Use of antibiotics was recognized as any systemic antibiotic within 72 h after the procedure. The number of procedures per year was calculated by dividing the total number of procedures by the follow-up duration in years.
Statistical analyses
Continuous variables were expressed as means ± standard deviations or as medians with quartiles if normality was not presumed. Categorical variables were reported as counts and percentages. Student’s t test or Mann–Whitney U tests were performed to test the association between the clinical success of interventional bronchoscopy and continuous variables, whereas the Chi-square test or Fisher’s exact test (if expected value ≤ 5) was performed for categorical variables. Univariate and multivariate logistic regressions were conducted with statistically significant predictors of the clinical outcome. Levels of C-reactive protein (CRP) were normalized by log10 transformation in the regression analysis. The significance level of all analyses was set at a P value < 0.05. Statistical analysis was conducted using SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA).
Results
Patients and baseline characteristics
Out of 718 patients with central airway stenosis that underwent interventional bronchoscopic procedures from January 2010 to April 2019, 119 patients (67 males and 52 females) were diagnosed with SCAS and met the criteria for enrollment in the study (Fig. 1). The overall median follow-up time was 53.7 (31.5, 77.0) months, of which 67.9 (42.2, 88.0) and 32.8 (23.5, 53.0) months in the successful and unsuccessful group, respectively. At last follow-up, 75 (63%) patients successfully maintained airway patency for over 12 months without symptoms. The baseline patient characteristics are provided in Table 1.
Fig. 1.
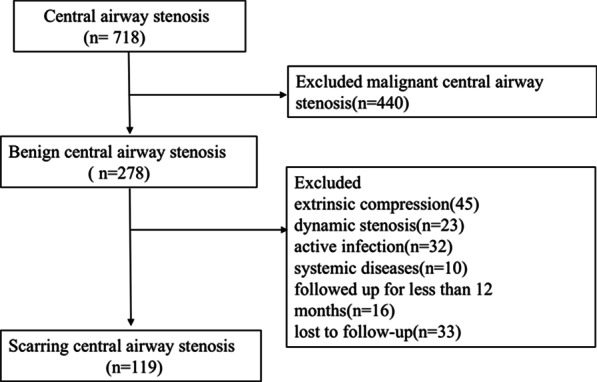
The flow chart of patients’ enrollment
Table 1.
Patients’ demographics
| Characteristic | All (n = 119) | Successful (n = 75) | Unsuccessful (n = 44) | P value |
|---|---|---|---|---|
| Age (year) | 45 (28, 56) | 43 (24, 55) | 50 (34, 59) | 0.034* |
| Female sex—no. (%) | 52 (43.7) | 39 (52.0) | 13 (29.5) | 0.017* |
| Smoking status | 0.035* | |||
| Current | 26 (21.9) | 11 (14.7) | 15 (34.1) | |
| Former | 21 (17.6) | 13 (17.3) | 8 (18.2) | |
| Nonsmoker | 72 (60.5) | 51 (68.0) | 21 (47.7) | |
| Underlying lung disease | ||||
| COPD | 8 (6.7) | 6 (8.0) | 2 (4.5) | 0.709 |
| Asthma | 4 (3.4) | 2 (2.7) | 2 (4.5) | 0.626 |
| Lung cancer | 9 (7.6) | 6 (8.0) | 3 (6.8) | 1.000 |
| Bronchiectasis | 3 (2.5) | 3 (4.0) | 0 | 0.295 |
| Symptom | ||||
| Cough | 51 (42.9) | 36 (48.0) | 15 (34.1) | 0.139 |
| Fever | 6 (5.0) | 4 (5.3) | 2 (4.5) | 1.000 |
| Dyspnea | 108 (90.8) | 68 (90.7) | 40 (90.9) | 1.000 |
| Chest pain | 4 (3.4) | 1 (1.3) | 3 (6.8) | 0.142 |
| WBC (× 109/l) | 6.40 (5.10, 7.60) | 5.90 (5.24, 7.40) | 6.65 (4.68, 8.35) | 0.725 |
| Neutrophil percentage (%) | 64.8 (56.6, 71.0) | 64.5 (56.6, 70.0) | 65.4 (56.8, 72.0) | 0.928 |
| CRP (mg/l) | 2.5 (0.8, 7.8) | 2 (0.6, 4.9) | 5 (1.1, 10.5) | 0.027* |
| ESR (mm/h) | 14 (8, 24) | 12 (7, 19) | 17.5 (8, 30.8) | 0.130 |
| ABG | 0.460 | |||
| Type I respiratory failure | 3 (2.8) | 3 (4.8) | 0 (0) | |
| Type II respiratory failure | 4 (3.7) | 2 (3.2) | 2 (4.5) | |
| CO2 retention | 9 (8.4) | 4 (6.3) | 5 (11.4) | |
| FEV1/pred (%) | 52.9 ± 20.2 | 54.9 ± 20.1 | 48.4 ± 20.1 | 0.198 |
| PEF/pred (%) | 35.7 (18.6, 63.1) | 39.0 (19.4, 60.7) | 24.4 (15.7, 69.2) | 0.494 |
| FVC/pred (%) | 79.3 ± 18.8 | 80.8 ± 19.2 | 76.0 ± 17.9 | 0.312 |
| FEV1/FVC (%) | 57.8 (38.4, 71.0) | 60.1 (38.0, 71.0) | 48.1 (39.2, 75.5) | 0.666 |
Data were expressed as count (percentage), mean ± SD or median (interquartile range) as appropriate
COPD chronic obstructive pulmonary diseases, FEV1 forced expiratory volume in one second, PEF peak expiratory flow, FVC forced vital capacity, CRP C-reactive protein, ESR erythrocyte sedimentation rate, WBC white blood cell, CO2 Carbon dioxide, ABG arterial blood gas
*p < 0.05
There were significant differences in terms of age (P = 0.034), gender (P = 0.017), smoking status (P = 0.035), and CRP level (P = 0.027) between the successful and unsuccessful groups. The median age of the unsuccessful group was 50 (34,59) years, which was significantly older than that of the successful group (odds ratio [OR] 1.03, 95% confidence interval [CI] 1.00–1.05, P = 0.037). Female gender tended to be a protective factor for therapeutic failure compared with male gender (OR 0.39, 95% CI 0.18–0.85, P = 0.019). In comparison with nonsmokers, current smokers had higher odds of unsuccessful therapeutic bronchoscopy (OR 3.31, 95% CI 1.31–8.39, P = 0.012). Importantly, there was no significant difference in odds of success between former smokers and nonsmokers (OR 1.50, 95% CI 0.54–4.13, P = 0.439). Univariate logistic regression analysis showed that elevated CRP increased the risk of therapeutic failure (OR 1.90, 95% CI 1.01–3.57, P = 0.046). No significant differences were observed in symptoms, underlying lung disease, white blood cell count, neutrophil percentage, erythrocyte sedimentation rate, pulmonary function test, and arterial blood gas between the two groups.
Airway stenosis characteristics
The airway stenosis characteristics are summarized in Table 2. The most common etiology of scarring airway stenosis was tracheal intubation or tracheostomy (n = 60, 50.4%), followed by tuberculosis (n = 32, 26.9%), surgical resection (n = 16, 13.4%), unknown reason (n = 7, 5.9%), radiation (n = 2, 1.7%), and trauma (n = 2, 1.7%), without predominance in the two groups. The degree of airway stenosis in the patients clustered between 71 and 99%, while occlusive lesions accounted for 10.9%. There were no significant differences in etiology, stenosis degree, multiple locations of airway stenosis, and airway stenosis length between the successful and unsuccessful groups. Stenosis was most commonly located in the subglottic area (26.9%) and tracheal regions (39.5%), corresponding to the most common etiology, tracheal intubation or tracheostomy. Compared to other stenosis locations, subglottic stenosis was more refractory (OR 6.50, 95% CI 2.67–15.83, P < 0.001).
Table 2.
Airway stenosis and interventional techniques characteristics
| Characteristic | All (n = 119) | Successful (n = 75) | Unsuccessful (n = 44) | P value |
|---|---|---|---|---|
| Etiology | 0.057 | |||
| Tracheal intubation or tracheostomy | 60 (50.4) | 34 (45.4) | 26 (59.1) | |
| Surgery | 16 (13.4) | 10 (13.3) | 6 (13.6) | |
| Trauma | 2 (1.7) | 1 (1.3) | 1 (2.3) | |
| TB | 32 (26.9) | 26 (34.7) | 6 (13.6) | |
| Radiation | 2 (1.7) | 0 | 2 (4.6) | |
| Unknown | 7 (5.9) | 4 (5.3) | 3 (6.8) | |
| Stenosis degree | 0.663 | |||
| II | 33 (27.7) | 20 (26.7) | 13 (29.6) | |
| III | 73 (61.3) | 48 (64.0) | 25 (56.8) | |
| IV | 13 (10.9) | 7 (9.3) | 6 (13.6) | |
| Location | ||||
| Subglottis | 32 (26.9) | 10 (13.3) | 22 (50.0) | < 0.001* |
| Trachea | 47 (39.5) | 33 (44.0) | 14 (31.8) | 0.189 |
| Right main bronchus | 16 (13.4) | 11 (14.7) | 5 (11.4) | 0.610 |
| Left main bronchus | 29 (24.4) | 22 (29.3) | 7 (15.9) | 0.100 |
| Intermediate bronchus | 4 (3.4) | 3 (4.0) | 1 (2.3) | 1.000 |
| Multilocation | 0.466 | |||
| Yes | 8 (6.7) | 4 (5.3) | 4 (9.1) | |
| No | 111 (93.3) | 71 (94.7) | 40 (90.9) | |
| Length | 0.366 | |||
| < 1 cm | 5 (4.8) | 4 (6.1) | 1 (2.6) | |
| 1–3 cm | 69 (66.3) | 46 (69.7) | 23 (60.5) | |
| ≥ 3 cm | 30 (28.9) | 16 (24.2) | 14 (36.8) | |
| Electrocautery | 0 (0, 1) | 0 (0, 1) | 1 (0, 2) | 0.089 |
| Laser | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0.006* |
| Mechanical dilation | 8 (4, 17) | 8 (4, 17) | 7 (4, 14) | 0.602 |
| Cryotherapy | 4 (2, 7) | 4 (2, 7) | 4 (2, 8) | 0.820 |
| Diprospan injection | 1 (0, 3) | 1 (0, 3) | 1 (0, 2) | 0.413 |
| Stent | < 0.001* | |||
| Yes | 23 (19.3) | 7 (9.3) | 16 (36.4) | |
| No | 96 (80.7) | 68 (90.7) | 28 (63.6) | |
| T-tube | 0.010* | |||
| Yes | 7 (5.9) | 1 (1.3) | 6 (13.6) | |
| No | 112 (94.1) | 74 (98.7) | 38 (86.4) | |
| Antibiotics | 0.526 | |||
| Yes | 55 (46.2) | 33 (44.0) | 22 (50.0) | |
| No | 64 (53.8) | 42 (56.0) | 22 (50.0) | |
| Time to intervention (days) from diagnosis | 22 (7, 72) | 22 (8, 63) | 28 (7, 92) | 0.884 |
| Previous interventional treatment | 0.022* | |||
| Yes | 34 (28.6) | 16 (21.3) | 18 (40.9) | |
| No | 85 (71.4) | 59 (78.7) | 26 (59.1) | |
| Number of procedures per year | 1 (0.5, 1.7) | 0.8 (0.4, 1.2) | 1.5 (0.8, 2.6) | < 0.001* |
| Interval to second procedure (days) | 21 (9, 342) | 21 (9, 269) | 25 (9, 400) | 0.934 |
| Stenosis degree change after first intervention (%) | 45 (30, 60) | 40 (20, 60) | 50 (38, 70) | 0.140 |
Data were expressed as count (percentage) or median (interquartile range) as appropriate
TB tuberculosis
*p < 0.05
Interventional bronchoscopic modalities
The associations of the procedural modalities and long-term outcome were listed in Table 2. The total number of therapeutic interventions performed during follow-up was 4 (2, 7) in the successful group and 5 (2, 7) in the unsuccessful group. Except for laser (P = 0.006), there were no differences in the use of electrocautery, mechanical dilation, cryotherapy, diprospan injection, or antibiotics between the two groups. The association with laser disappeared in the univariate analysis (OR 1.68, 95% CI 0.95–2.97, P = 0.074). Since there were more patients with tuberculosis in the successful group than in the unsuccessful group (34.7% vs 13.6%), subgroup analysis confined to patients without tuberculosis (n = 87) was performed and revealed less prescription of antibiotics in the successful group (38.8% vs 50.0%). Nevertheless, the difference was not statistically significant (P = 0.295). Patients with stent (OR 5.55, 95% CI 2.06–14.96, P = 0.001) or T-tube implantation (OR 11.68, 95% CI 1.36–100.59, P = 0.025) were more likely to be associated with unsuccessful outcome. The number of procedures per year was significantly higher in the unsuccessful group than that in the successful group, and was associated with higher odds of failure (OR 2.04, 95% CI 1.36–3.06, P = 0.001).
Twenty-three (19.3%) patients received 26 stent placements, all of which were silicone stents, including 4 Y-shaped and 22 straight stents. Sixteen stents were removed after 15.4 ± 8.7 months. Seven and 16 patients with stent implantation were in the successful group and unsuccessful group, respectively. All those in the successful group had their stents removed, whereas 6/16 (37.5%) had their stents removed in the unsuccessful group (P = 0.007). There was no difference in the duration from stent placement to removal between the two groups (16.4 ± 5.4 m vs 14.6 ± 10.9 months, respectively).
Variables associated with success of therapeutic bronchoscopy
We assessed all the above factors that were associated with long-term outcome using multivariate logistic regression analysis (Additional file 1: Table S1). Current smoker status (OR 5.70, 95% CI 1.35–24.17, P = 0.018), subglottic stenosis (OR 4.35, 95% CI 1.31–14.46, P = 0.017), and stent implantation (OR 4.96, 95% CI 1.33–18.48, P = 0.017) were associated with lower odds of success after controlling for other variables.
To control the confounding effect of follow-up time, we performed a subgroup analysis including 57 patients from July 2014 to July 2018 (Additional file 1: Table S2). The follow-up time was comparable between the two groups (45.5 vs 39.5 months, respectively). The risk of subglottic stenosis and stent implantation remained independent, while that of current smoking status was significant at the 10% level.
Complications
Over the 577 interventional bronchoscopies, most of the therapies were performed without significant complications (90.5%). The complications were all manageable, including bleeding requiring adrenaline spraying or electrocoagulation (2.8%), pneumomediastinum or pneumothorax (0.4%), CO2 retention (0.2%), fever (0.4%), secretion retention (0.6%), severe bronchial laceration (2.1%), stent migration (1.6%), and stent-related granulation hyperplasia (1.4%). There was no interventional bronchoscopy-related death at our institution.
Discussion
Interventional bronchoscopy can rapidly improve short-term efficacy by relieving symptoms and airway obstruction [13]. However, few studies have explored the long-term outcomes and prognostic factors of therapeutic bronchoscopy. In this study, 119 patients with SCAS from January 2010 to April 2019 were retrospectively reviewed. We found that 63.0% of the patients maintained long-term stability. Long-term failure of bronchoscopic interventions for SCAS was associated with current smoker status, subglottic stenosis, and stent implantation.
In this study, the female gender was associated with higher odds of clinical success of interventional bronchoscopy. A better therapeutic effect was observed in females. Similarly, in a series of 115 patients with postintubation tracheal stenosis (PITS), Freitas et al. [14] found that simple PITS occurred more frequently in females, whereas complex PITS was more common in males. These findings imply that the therapeutic outcome varies with gender in SCAS.
Another significant finding in the study is that cigarette smoking is related to a higher probability of delayed long-term efficacy. Compared to nonsmokers, current smokers had five times the odds of unsuccessful therapeutic bronchoscopy. Notably, such a difference disappeared between former smokers and nonsmokers. A previous study of patients with laryngotracheal stenosis reported that smoking was associated with tracheostomy dependence and shorter intervals between therapeutic procedures [15]. In addition to benign airway stenosis, Giovacchini et al. [16] found that nonsmokers and former smokers had a higher success rate of therapeutic bronchoscopy than current smokers in malignant conditions. Several studies had proved that smoking could increase airway epithelial inflammation and induce pathologic wound healing [17, 18]. Similarly, it is very likely that smoking could aggravate the injury to the airway mucosa, leading to worse outcomes in SCAS. In accordance with other studies showing worse outcomes in active smokers [19, 20], we confirm cigarette smoking is correlated with poor post-procedural prognosis, and recommend that smoking cessation should be incorporated into future guidelines regarding the management of SCAS.
The most common cause of SCAS in this study was stenosis after tracheal intubation or tracheostomy. A previous study reported that tuberculosis was the most common cause of SCAS in Chinese patients, whereas tracheal intubation or tracheostomy were more common in western countries [21, 22]. We hypothesize that the change in the etiology spectrum is primarily due to the decreased incidence of tuberculosis, which could be attributed to standardized Bacillus Calmette–Guérin vaccination and anti-tuberculosis treatment. Accordingly, the Global Tuberculosis Report 2017 indicated that the global tuberculosis incidence was decreasing every year [23].
Additionally, we found that subglottic stenosis was associated with a significantly lower probability of long-term therapeutic success, in agreement with our clinical experiences. The recurrence rate of subglottic stenosis requiring recurrent endoscopic therapy had been reported to be as high as 40–70% [24]. Hseu et al. conducted a 10-year review of 92 adults with subglottic stenosis, and found that 55% of patients required multiple procedures [25]. Nair et al. [26] reported that the incidence of subglottic decannulation was 68.8% lower than those of glottic and supraglottic stenosis. The subglottic area borders from 5 to 10 mm beneath the vocal folds to the inferior rim of the cricoid cartilage, and the diameter of the cricoid is equivalent to the left main bronchus [27]. Its narrow lumen and special anatomical architecture might make it predisposed to temporized success. Gelbard et al. [28] reported that the iatrogenic etiology of prolonged intubation was one of the most important causes of subglottic stenosis in adults. Therefore, intubation with a proper-sized endotracheal tube, avoiding excessive intra-cuff pressure, and opportune ventilator weaning should be emphasized to minimize injury to the subglottic area.
In this study, for the first time, we demonstrated that CRP might be a candidate predictor of interventional therapeutic outcome. Elevated CRP levels were associated with decreased odds of therapeutic success. CRP is an acute-phase protein and sensitive biomarker of systemic inflammation, primarily secreted by hepatocytes upon stimulation from interleukin (IL)-6 and TNF-α [29, 30]. A previous animal study had confirmed that repeated intubation could result in significant tracheal injury and elevated IL-6 levels both in serum and tracheal tissues [31]. Overexpressed IL-6 upregulates its downstream targets including JAK1, STAT3, RAF1, and ELK1, and induces fibroblastic proliferation and excessive collagen deposition, leading to scar formation [32]. In addition, CRP had been considered as a biomarker of renal and cardiac inflammation and fibrosis [33, 34]. Hence, we speculate that CRP levels correlate positively with the process of airway injury and scar formation, and may serve as a potential serologic marker of SCAS. Yet the underlying association is incompletely understood, which warrants further investigation.
Combined modalities were applied in most of our cases. Thermal therapy has been reported to cause significant granulation tissue growth and cartilage damage compared with mechanical dilation and cryotherapy [35–38]. Thus, we avoided using these modalities where possible to minimize damage to the airway. As scar tissue is resistant to cryotherapy [39], a high frequency electric needle knife or laser was used to cut the scar open radially only when it was too difficult to dilate. Therefore, for non-stent interventional modalities, balloon or rigid bronchoscopy dilation and cryotherapy were the most common techniques we used. In terms of the stent, 23 (19.3%) patients received silicone stent implantation, and the rate of stent implantation was significantly higher in the unsuccessful group. An explanation for this lies in the notion that the patients who were considered for stent implantation in our cohort were considerably sicker and had poor condition or recurrent and intractable stenosis, making the intervention more likely to fail. Moreover, stent implantation may increase the extent of injury and the length of stenosis [38]. Long-term stent implantation is known to be associated with various complications, such as sputum retention, stent migration, and granulation tissue formation [38, 40, 41], which could induce airway restenosis and demand close bronchoscopic surveillance. Intractable stenosis often requires more frequent interventions and multiple procedures to maintain airway patency, and conversely, frequent interventions and multiple procedures can induce secondary airway damage and restenosis, causing therapeutic failure.
Long-term therapeutic success was achieved in 63.0% of the patients in our study, which was identical to previous data reported by Wang et al. [21]. Overall, 90.5% of procedures were conducted without significant complications, and there was no interventional bronchoscopy-related death at our institution. All the above indicate that interventional bronchoscopy is safe and effective for scarring airway stenosis. Our study identified predictors of poor outcome of interventional bronchoscopy, highlighting the crucial role of interventional procedures in selected cases. For those who are unlikely to benefit from the intervention, surgical therapy may be considered in clinical decision-making.
Our study had several limitations. First, it was a retrospective study. Some missing data were inevitable, even if we tried to avoid this eventuality when collecting data. Second, the analysis only included treatment information at our institution. Information of previous treatment(s) at other institutions was not collected due to the lack of detailed records. This might have biased the influence of therapies. Third, the study was conducted at a single institution. A multicenter study is necessary to validate our findings in the future. Finally, the difference in follow-up time between the two groups may have affected long-term success to an extent. We have tried our best to control the potential confounding by including follow-up time as a covariate and conducting subgroup analysis. Further research is needed to assess whether the predictors of long-term therapeutic bronchoscopy success for SCAS in our study hold true in prospective studies.
Conclusions
In conclusion, interventional bronchoscopy was safe and effective for patients with SCAS. Patients who were current smokers, had subglottic stenosis, and those requiring stent implantation were more likely to experience delayed long-term effectiveness. Smoking cessation should be recommended to optimize the outcomes of therapeutic bronchoscopy.
Supplementary Information
Additional file 1. Variables Associated with Therapeutic Bronchoscopy.
Acknowledgements
We thank all the patients for their full cooperation throughout treatments at our hospital and for giving consents to this study. We would also particularly like to thank interventional pulmonology nurses Yu-Hong Gong, Lin Guo, and Ya-Li Jia for their assistance and knowledge in the procedures.
Abbreviations
- CI
Confidence interval
- CO2
Carbon dioxide
- CRP
C-reactive protein
- IL
Interleukin
- OR
Odds ratio
- PITS
Postintubation tracheal stenosis
- SCAS
Scarring central airway stenosis
Authors’ contributions
KYS designed the study, collected and analyzed the data, and wrote the manuscript. HZ helped to interpret the data and draft the manuscript. GFW designed the study and revised the manuscript. HZ, WZ, and YC helped interpreted the data. GFW, HZ, WZ, and YC undertook the interventional bronchoscopies. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The Ethics Committee of Peking University First Hospital approved this study and oral informed consent (2020209). Oral informed consent was obtained from all patients. Access to the patients’ data was permitted by the Committee of Peking University First Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J. 2007;30:7–12. doi: 10.1183/09031936.00132804. [DOI] [PubMed] [Google Scholar]
- 2.Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278–1297. doi: 10.1164/rccm.200210-1181SO. [DOI] [PubMed] [Google Scholar]
- 3.Murgu SD, Egressy K, Laxmanan B, et al. Central airway obstruction: benign strictures, tracheobronchomalacia, and malignancy-related obstruction. Chest. 2016;150:426–441. doi: 10.1016/j.chest.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Barros Casas D, Fernández-Bussy S, Folch E, et al. Non-malignant central airway obstruction. Arch Bronconeumol. 2014;50:345–354. doi: 10.1016/j.arbres.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Krajc T, Janik M, Benej R, et al. Urgent segmental resection as the primary strategy in management of benign tracheal stenosis. A single center experience in 164 consecutive cases. Interact Cardiovasc Thorac Surg. 2009;9:983–989. doi: 10.1510/icvts.2009.213215. [DOI] [PubMed] [Google Scholar]
- 6.Dalar L, Karasulu L, Abul Y, et al. Bronchoscopic treatment in the management of benign tracheal stenosis: choices for simple and complex tracheal stenosis. Ann Thorac Surg. 2016;101:1310–1317. doi: 10.1016/j.athoracsur.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Mahmood K, Wahidi MM, Thomas S, et al. Therapeutic bronchoscopy improves spirometry, quality of life, and survival in central airway obstruction. Respiration. 2015;89:404–413. doi: 10.1159/000381103. [DOI] [PubMed] [Google Scholar]
- 8.Liang W, Hu P, Guo W, et al. Appropriate treatment sessions of flexible bronchoscopic balloon dilation for patients with nonmalignant central airway stenosis. Ther Adv Respir Dis. 2019;13:1753466619831966. doi: 10.1177/1753466619831966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope. 2009;119:272–283. doi: 10.1002/lary.20056. [DOI] [PubMed] [Google Scholar]
- 10.Sratakos G, Chiotis D, Zisis C, et al. Long-term outcomes of patients with benign tracheal stenosis after multidisciplinary management. Eur Respir J. 2011;38(Suppl 55):p613. [Google Scholar]
- 11.Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg. 2009;35:429–433. doi: 10.1016/j.ejcts.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Myer CM, 3rd, O'Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994;103:319–323. doi: 10.1177/000348949410300410. [DOI] [PubMed] [Google Scholar]
- 13.Okiror L, Jiang L, Oswald N, et al. Bronchoscopic management of patients with symptomatic airway stenosis and prognostic factors for survival. Ann Thorac Surg. 2015;99:1725–1730. doi: 10.1016/j.athoracsur.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 14.Morais A, Fernandes G, Magalhães A. The role of interventional bronchoscopy in the management of post-intubation tracheal stenosis: a 20-year experience. Pulmonology. 2019;S2531–0437(19):30222–30223. doi: 10.1016/j.pulmoe.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Gadkaree SK, Pandian V, Best S, et al. Laryngotracheal stenosis: risk factors for tracheostomy dependence and dilation interval. Otolaryngol Head Neck Surg. 2017;156:321–328. doi: 10.1177/0194599816675323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovacchini CX, Kessler ER, Merrick CM, et al. Clinical and radiographic predictors of successful therapeutic bronchoscopy for the relief of malignant central airway obstruction. BMC Pulm Med. 2019;19:219. doi: 10.1186/s12890-019-0987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann RF, Zarrintan S, Brandenburg SM, et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013;14(1):97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Yu HM, Zhou XD, et al. Cigarette smoke induces mucin hypersecretion and inflammatory response through the p66shc adaptor protein-mediated mechanism in human bronchial epithelial cells. Mol Immunol. 2016;69:86–98. doi: 10.1016/j.molimm.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Gadkaree SK, Gelbard A, Best SR, et al. Outcomes in bilateral vocal fold immobility: a retrospective cohort analysis. Otolaryngol Head Neck Surg. 2018;18:194599818800462. doi: 10.1177/0194599818800462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282–1298. doi: 10.1378/chest.14-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Zhang J, Qiu XJ, et al. Scarring airway stenosis in Chinese adults: characteristics and interventional bronchoscopy treatment. Chin Med J (Engl) 2018;131:276–281. doi: 10.4103/0366-6999.223850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman NA, Fruchter O, Shitrit D, et al. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg. 2010;5:2. doi: 10.1186/1749-8090-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global tuberculosis report 2017. Geneva: World Health Organization; 2017.
- 24.Cataneo DC, Ximenes AMG, Cataneo AJM. Mitomycin C in the endoscopic treatment of tracheal stenosis: a prospective cohort study. J Bras Pneumol. 2018;44:486–490. doi: 10.1590/s1806-37562017000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hseu AF, Benninger MS, Haffey TM, et al. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope. 2014;124:736–741. doi: 10.1002/lary.24410. [DOI] [PubMed] [Google Scholar]
- 26.Nair S, Nilakantan A, Sood A, et al. Challenges in the management of laryngeal stenosis. Indian J Otolaryngol Head Neck Surg. 2016;68:294–299. doi: 10.1007/s12070-015-0936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thurnher D, Moukarbel RV, Novak CB, et al. The glottis and subglottis: an otolaryngologist's perspective. Thorac Surg Clin. 2007;17:549–560. doi: 10.1016/j.thorsurg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Gelbard A, Francis DO, Sandulache VC, et al. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2015;125:1137–1143. doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/S0065-2776(08)60379-X. [DOI] [PubMed] [Google Scholar]
- 30.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshodi A, Dysart K, Cook A, et al. Airway injury resulting from repeated endotracheal intubation: possible prevention strategies. Pediatr Crit Care Med. 2011;12:e34–e39. doi: 10.1097/PCC.0b013e3181dbeb28. [DOI] [PubMed] [Google Scholar]
- 32.Ghazizadeh M, Tosa M, Shimizu H, et al. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol. 2007;127:98–105. doi: 10.1038/sj.jid.5700564. [DOI] [PubMed] [Google Scholar]
- 33.Li ZI, Chung AC, Zhou L, et al. C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab Invest. 2011;91(6):837–851. doi: 10.1038/labinvest.2011.42. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Zhang YY, Huang XR, et al. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease. Hypertension. 2010;55(4):953–960. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wang T, Wang J, et al. Effect of three interventional bronchoscopic methods on tracheal stenosis and the formation of granulation tissues in dogs. Chin Med J (Engl) 2010;123:621–627. [PubMed] [Google Scholar]
- 36.Verkindre C, Brichet A, Maurage CA, et al. Morphological changes induced by extensive endobronchial electrocautery. Eur Respir J. 1999;14:796–799. doi: 10.1034/j.1399-3003.1999.14d12.x. [DOI] [PubMed] [Google Scholar]
- 37.Dunlap DG, Ravenel J, Sechrist J, et al. Interventional therapies for central airways. J Thorac Imaging. 2019;34:W49–W59. doi: 10.1097/RTI.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 38.Rea F, Callegaro D, Loy M, et al. Benign tracheal and laryngotracheal stenosis: surgical treatment and results. Eur J Cardiothorac Surg. 2002;22:352–356. doi: 10.1016/S1010-7940(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 39.DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic cryotherapy. Clinical applications of the cryoprobe, cryospray, and cryoadhesion. Ann Am Thorac Soc. 2016;13:1405–1415. doi: 10.1513/AnnalsATS.201601-062FR. [DOI] [PubMed] [Google Scholar]
- 40.Ko PJ, Liu CY, Wu YC, et al. Granulation formation following tracheal stenosis stenting: influence of stent position. Laryngoscope. 2009;119:2331–2336. doi: 10.1002/lary.20615. [DOI] [PubMed] [Google Scholar]
- 41.Shin JH, Song HY, Shim TS. Management of tracheobronchial strictures. Cardiovasc Intervent Radiol. 2004;27:314–324. doi: 10.1007/s00270-003-0134-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Variables Associated with Therapeutic Bronchoscopy.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


