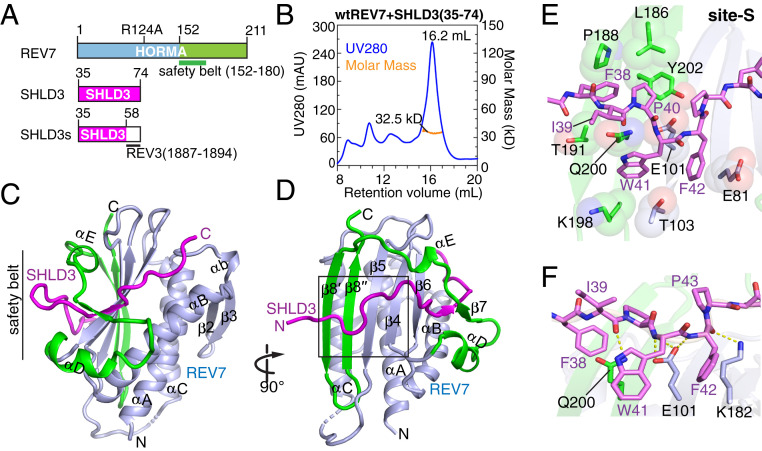
Fig. 1.
Crystal structure of SHLD3s–REV7 monomer complex reveals safety-belt topology and site-S interface. (A) Schematic drawing of human REV7 and SHLD3s fusion protein. The safety-belt segment of REV7 spans residues 152 to 180. (B) Purification of the SHLD3 (35 to 74)–REV7 monomer complex on an S200 gel filtration column. The major peak exhibited a mol. wt. = 32.5 kDa by SEC-MALS. (C and D) Two views of the overall structure of the SHLD3s–REV7 monomer complex. The N- and C-terminal halves of REV7 monomer are colored in light blue and green, respectively, while SHLD3s is colored in magenta. A black box highlights the site-S region in D. (E and F) Hydrophobic (E) and hydrogen bonding (F) interactions involving site-S. As shown in E, the bulky side chain of SHLD3 Phe-38 wedges into the hydrophobic pocket lined by REV7 residues Leu-186, Pro-188, Thr-191, and Tyr-202, while SHLD3 Trp-41 and Phe-42 stack tightly with the side chains of Glu-81, Glu-101, Thr-103, Lys-198, and Gln-200 of REV7. As shown in F, the backbones of SHLD3 Ile-39, Trp-41, Phe-42, and Pro-43 further interacts with the side chains of REV7 residues Glu-101, Lys-182, and Leu-173, and Ala-174 by hydrogen bonding.