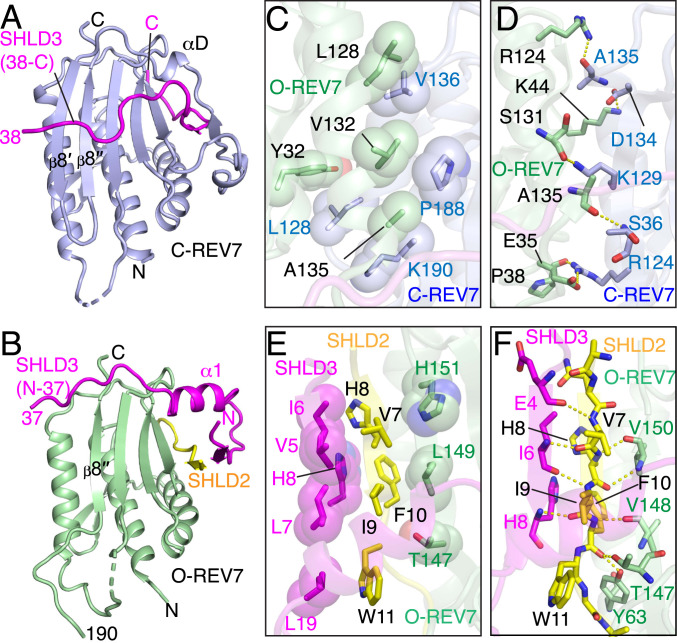
Fig. 3.
Comparison of closed C-REV7 and open O-REV7 conformations in the SHLD2.3–REV74 complex and intermolecular contacts. (A and B) Closed C-REV7 (A) and open O-REV7 (B) conformations in the SHLD2.3–REV74 complex. (C and D) Dimeric interface between C-REV7 and O-REV7 in the SHLD2.3–REV74 complex is mediated by multiple intermolecular hydrophobic (C) and hydrogen bonding (D) interactions. Trp-32, Leu-128, Val-132, and Ala-135 of O-REV7 build up a hydrophobic core with Leu-128, Vla-136, Pro-188, and Lys-190 of C-REV7 (C). Both Arg-124 of O-REV7 and C-REV7 form hydrogen bonds with Ala-135 of C-REV7 and Glu-35 of O-REV7, respectively, highlighting the importance of Arg-124 that is shown in previous reports. Lys-44, Ser-131, and Ala-135 of O-REV7 form additional hydrogen bonds with Asp-134, Lys-129, and Ser-36, respectively (D). (E and F) SHLD2–SHLD3 alignment in the O-REV7 is mediated by multiple intermolecular hydrophobic (E) and hydrogen bonding (F) interactions. Thr-147, Leu-149, and His-151 of O-REV7 and Val-5, Ile-6, Leu-7 His-8, and Leu-19 of SHLD3 build a hydrophobic core with Val-7, His-8, Ile-8, Phe-10, and Trp-11 of SHLD2 (E). The β-sheet is further stabilized by multiple backbone hydrogen bonds, while the backbone carbonyl oxygen of SHLD2 Phe-10 is recognized by the side chains of REV7 Tyr-63 and Thr-147 by hydrogen bonding (F).