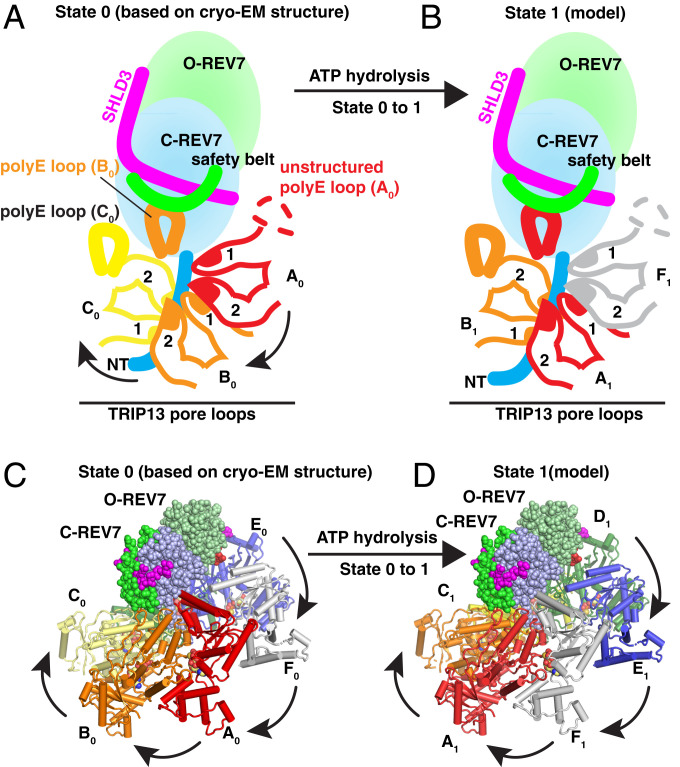
Fig. 8.
Model of SHLD2.3–REV7 dimer complex remodeling mediated by the ATP-driven translocation of the TRIP13 hexamer. (A and B)Schematic of the proposed remodeling mechanism of TRIP13-mediated SHLD2.3–REV7 dimer. The fingers of TRIP13 grip the REV7NT threaded segment tightly and the translocation of TRIP13 monomers draws the thread from REV7 into the channel in stepwise manner. (Cand D) Models of the SHLD2.3–REV74–TRIP13 complexes in basal state 0 and basal state 1 (before and after the first catalytic cycle, see more details in Movie S1). For clarity, only one copy of SHLD2.3–REV7 dimer is shown in a sphere representation. In basal state 0 (C), TRIP13 monomers A0, B0, and C0 hold the C-REV7NT, while monomer E0 contacts O-REV7. As shown in D, the first cycle of ATP hydrolysis occurs in monomer E0, which transforms from a compact ATP-bound state to the flexible apo-state E1; the neighboring seam monomer F0 binds one ATP molecule to adopt the ATP-bound F1 state. These structural changes cause F1 to climb to the top of the AAA+ spiral to push an anticlockwise rotation of the SHLD2.3–REV7 dimer, which renders O-REV7 to form new contacts with monomer D1.