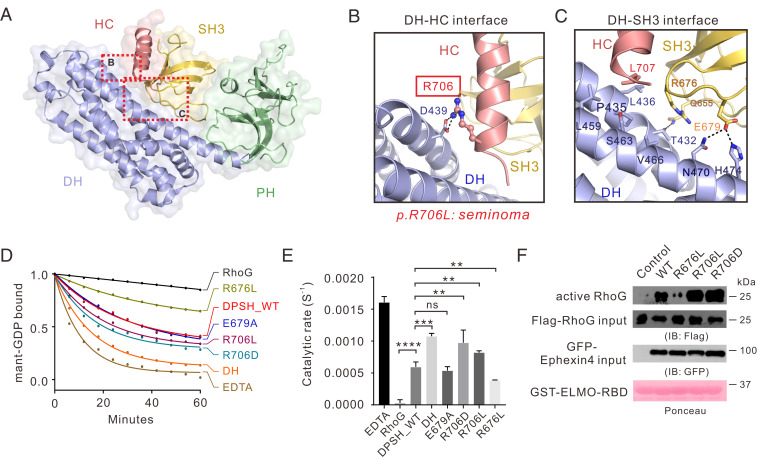
Fig. 2.
Interdomain interactions in Ephexin4DPSH. (A) The combined surface and ribbon representations of the Ephexin4DPSH structure showing that the DH, PH, and SH3–HC units couple tightly with each other. The DH–HC and DH–SH3 interfaces are indicated as dotted boxes. (B and C) Zoomed-in view of the DH–HC (B) and DH–SH3 (C) interface. The key residue R706 associated with cancer are indicated by the red box and the side chain of R706 is shown in the stick mode. (D) Representative in vitro GEF assays showing that the R706D mutation significantly increased the Ephexin4DPSH GEF activity for RhoG compared to WT. (E) Quantification of GEF assays testing the role of residues at DH–HC and DH–SH3 interfaces in regulation of Ephexin4 activity shown in D. Values are expressed as mean ± SD. ns, not significant, ****P < 0.0001, ***P < 0.001, **P < 0.01. All GEF assays in this study were performed using three independent protein preparations with at least duplicate measurements. (F) Either WT or mutant variants of Ephexin4 were cotransfected with Flag-tagged RhoG in HEK 293T cells. Active RhoG was coprecipitated with GST-ELMO2-RBD and detected by immunoblotting.