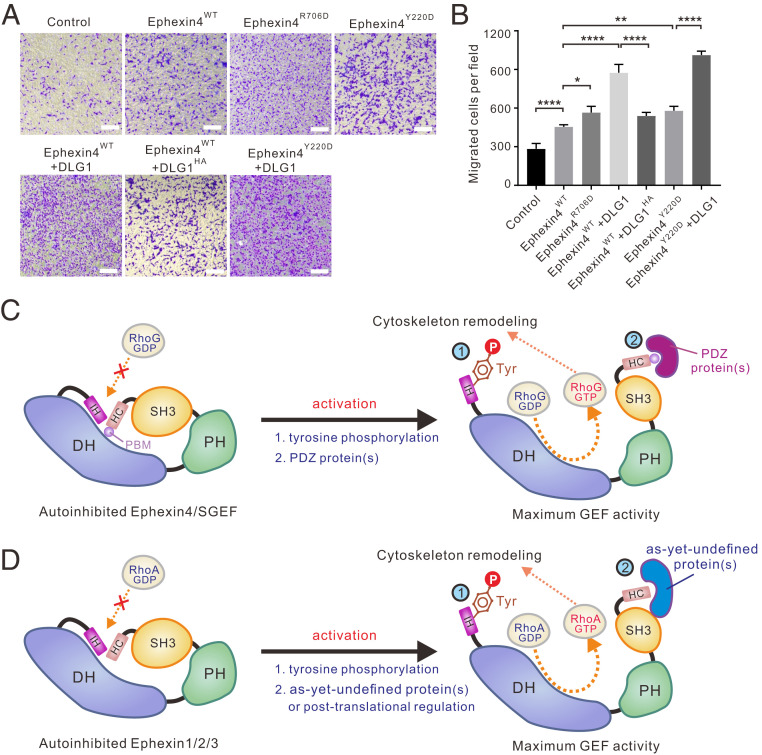
Fig. 5.
Relief of Ephexin4 autoinhibition promotes cell migration. (A) Transwell migration assays were performed to measure the cell migration activities of HeLa cells transfected with the indicated plasmids. (Scale bar: 20 μm.) (B) Quantification of cell migration abilities of the indicated plasmids described in A. Data are expressed as mean ± SD for each group from three independent experiments. ****P < 0.0001, **P < 0.01, *P < 0.1. (C and D) Depiction of the double inhibition and activation mechanisms in Ephexins and SGEF. The double inhibition involves both N-terminal (IH-mediated) and C-terminal (SH3–HC-mediated) inhibitory modes which prevents the small GTPases (e.g., RhoG and RhoA) from binding to the catalytic DH domain. Phosphorylation of a conserved tyrosine residue in IH relieves the N-terminal inhibition in Ephexins and SGEF. Binding of PDZ-containing protein(s) relieves the C-terminal inhibition in Ephexin4 and SGEF, which possess a PBM at the very C terminus (C) while, in other Ephexins (D), association with as-yet-undefined protein(s) or posttranslational modifications may contribute to the activation process.