FIGURE 1.
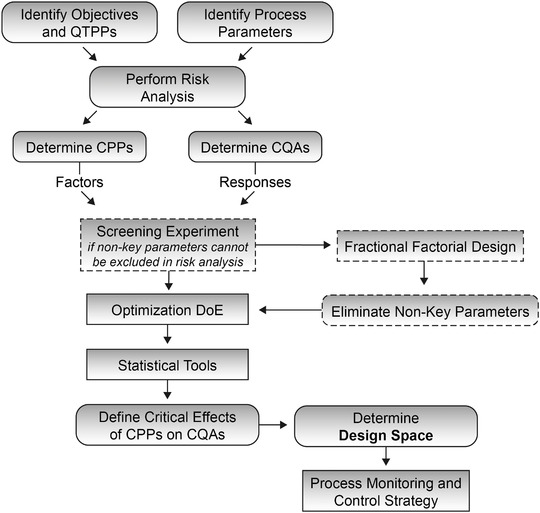
Schematic strategy for implementation of QbD and determination of a process design space in a biopharmaceutical production process. CPP, critical process parameter; CQA, critical quality attribute; DoE, design of experiments; QTPP, quality target product profile
